Successful Application of Tocilizumab in a Patient With Neoadjuvant Immunochemotherapy-Induced Cytokine Release Syndrome
- PMID: 39051558
- PMCID: PMC11270316
- DOI: 10.1002/cnr2.2145
Successful Application of Tocilizumab in a Patient With Neoadjuvant Immunochemotherapy-Induced Cytokine Release Syndrome
Abstract
Background: The expansion of preoperative immunochemotherapy has led to an increase in the number of patients with lung cancer receiving immune checkpoint inhibitors (ICIs). Therefore, oncologists should manage a variety of immune-related adverse events (irAEs). One of the rare, life-threatening, and recently proposed irAEs is cytokine release syndrome (CRS). Although the standard treatment of irAE is systemic administration of steroids, it has been suggested that tocilizumab may be an effective treatment option for CRS.
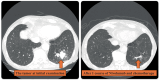
Case: This case describes a 69-year-old man with stage IIIA lung adenocarcinoma who received chemotherapy and nivolumab, which is an ICI, as neoadjuvant immunochemotherapy. After the first administration, the patient developed severe skin rash, fever, and arthralgia. We suspected irAEs and administered systemic steroids. However, fever and arthralgia did not improve, although the skin rash disappeared. These were also significant challenges for surgery. Noting the elevated levels of inflammatory cytokines, we consulted a rheumatologist. Finally, we decided to terminate neoadjuvant therapy after one cycle and administer tocilizumab. Tocilizumab dramatically improved the patient's symptoms and allowed him to undergo radical surgery. Pathological findings revealed that the patient achieved a major pathological response.
Conclusion: This indicates the potential effectiveness of early tocilizumab administration for ICI-induced CRS, even in mild cases.
Keywords: adenocarcinoma; cytokine release syndrome; neoadjuvant therapy; nivolumab; tocilizumab.
© 2024 The Author(s). Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
Yosuke Kawashima received personal fees from Taiho Pharmaceutical, Eli Lilly, Life Technologies Japan Ltd, Chugai Pharma, AstraZeneca, and Kyowa Kirin. Yukihiro Toi received personal fees from AstraZeneca, Chugai Pharma, Pfizer, Taiho Pharmaceutical, Kyowa Kirin, Bristol‐Myers Squibb, Ono Pharmaceutical, and MSD K.K. Shinsuke Yamanda received personal fees from AstraZeneca, Novartis, Sanofi, GSK, and Nippon Boehringer Ingelheim. Shunichi Sugawara received personal fees from AstraZeneca, Chugai Pharma, Pfizer, Taiho Pharmaceutical, Eli Lilly, Novartis, Kyowa Kirin, Bristol‐Myers Squibb, Ono Pharmaceutical, MSD K.K, and Nippon Boehringer Ingelheim. All other authors declare no conflict of interest.
Figures


References
-
- Shiraishi Y., Tokito T., Toyozawa R., et al., “Five Cases of Cytokine Release Syndrome in Patients Receiving Cytotoxic Chemotherapy Together With Nivolumab Plus Ipilimumab: A Case Report,” Journal of Thoracic Oncology 19, no. 2 (2024): 337–343. - PubMed
-
- Gödel P., Shimabukuro‐Vornhagen A., and von Bergwelt‐Baildon M., “Understanding Cytokine Release Syndrome,” Intensive Care Medicine 44 (2018): 371–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

