Analyzing the role of ferroptosis in ribosome-related bone marrow failure disorders: From pathophysiology to potential pharmacological exploitation
- PMID: 39052023
- PMCID: PMC11580388
- DOI: 10.1002/iub.2897
Analyzing the role of ferroptosis in ribosome-related bone marrow failure disorders: From pathophysiology to potential pharmacological exploitation
Abstract
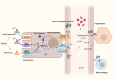
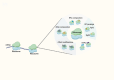
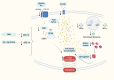
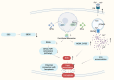
Within the last decade, the scientific community has witnessed the importance of ferroptosis as a novel cascade of molecular events leading to cellular decisions of death distinct from apoptosis and other known forms of cell death. Notably, such non- apoptotic and iron-dependent regulated cell death has been found to be intricately linked to several physiological processes as well as to the pathogenesis of various diseases. To this end, recent data support the notion that a potential molecular connection between ferroptosis and inherited bone marrow failure (IBMF) in individuals with ribosomopathies may exist. In this review, we suggest that in ribosome-related IBMFs the identified mutations in ribosomal proteins lead to changes in the ribosome composition of the hematopoietic progenitors, changes that seem to affect ribosomal function, thus enhancing the expression of some mRNAs subgroups while reducing the expression of others. These events lead to an imbalance inside the cell as some molecular pathways are promoted while others are inhibited. This disturbance is accompanied by ROS production and lipid peroxidation, while an additional finding in most of them is iron accumulation. Once lipid peroxidation and iron accumulation are the two main characteristics of ferroptosis, it is possible that this mechanism plays a key role in the manifestation of IBMF in this type of disease. If this molecular mechanism is further confirmed, new pharmacological targets such as ferroptosis inhibitors that are already exploited for the treatment of other diseases, could be utilized to improve the treatment of ribosomopathies.
Keywords: anemia; bone marrow‐failure disorders; ferroptosis; hematopoiesis; iron metabolism; pharmacology; ribosomopathies.
© 2024 The Author(s). IUBMB Life published by Wiley Periodicals LLC on behalf of International Union of Biochemistry and Molecular Biology.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
References
-
- Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide‐induced ferroptosis. Cell. 2018;172:409–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

