United States clinical practice experience with eculizumab in myasthenia gravis: symptoms, function, and immunosuppressant therapy use
- PMID: 39052039
- PMCID: PMC11377470
- DOI: 10.1007/s00415-024-12569-w
United States clinical practice experience with eculizumab in myasthenia gravis: symptoms, function, and immunosuppressant therapy use
Abstract
Background/objectives: The phase 3 REGAIN study and its open-label extension demonstrated the efficacy of the complement C5 inhibitor eculizumab in patients with treatment-refractory, acetylcholine receptor antibody-positive generalized myasthenia gravis (gMG). The aim of the ELEVATE study was to assess the effectiveness of eculizumab in clinical practice in adults with MG in the United States.
Methods: A retrospective chart review was conducted in adults with MG who initiated eculizumab treatment between October 23, 2017 and December 31, 2019. Outcomes assessed before and during eculizumab treatment using a pre- versus post-treatment study design included Myasthenia Gravis-Activities of Daily Living (MG-ADL) total scores; minimal symptom expression (MSE); physician impression of clinical change; minimal manifestation status (MMS); and concomitant medication use.
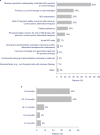
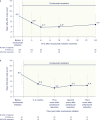
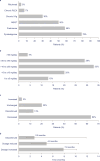
Results: In total, 119 patients were included in the study. A significant reduction was observed in mean MG-ADL total score, from 8.0 before eculizumab initiation to 5.4 at 3 months and to 4.7 at 24 months after eculizumab initiation (both p < 0.001). At 24 months after eculizumab initiation, MSE was achieved by 19% of patients. MMS or better was achieved by 30% of patients at 24 months. Additionally, 64% of patients receiving prednisone at eculizumab initiation had their prednisone dosage reduced during eculizumab treatment and 13% discontinued prednisone; 32% were able to discontinue nonsteroidal immunosuppressant therapy.
Discussion: Eculizumab treatment was associated with sustained improvements in MG-ADL total scores through 24 months in adults with MG. Prednisone dosage was reduced in approximately two-thirds of patients, suggesting a steroid-sparing effect for eculizumab.
Keywords: Activities of daily living; C5; Chart review; Clinical practice; Complement inhibition; Corticosteroid; Eculizumab; Immunosuppression; Myasthenia gravis.
© 2024. The Author(s).
Conflict of interest statement
Ali A. Habib has served as a medical advisor and speaker on behalf of Alexion, AstraZeneca Rare Disease, and argenx; he has received research support from Alexion, AstraZeneca Rare Disease, and Cabaletta Bio; and has received research support and honoraria from argenx, UCB, Pfizer, Genentech, Viela Bio/Horizon, Sanofi, Immunovant Inc., and Regeneron. Andrew J. Klink is a salaried employee of and owns stock in Cardinal Health, which received funding from Alexion, AstraZeneca Rare Disease for work performed on this study. Srikanth Muppidi has served on advisory board meetings for Alexion, AstraZeneca Rare Disease, argenx, Horizon Therapeutics, and Ra Pharmaceuticals (now UCB). Anju Parthan was an employee of Alexion, AstraZeneca Rare Disease and owned stock in AstraZeneca at the time the study was conducted and analyzed. S. Chloe Sader is an employee of Alexion, AstraZeneca Rare Disease and owns stock in AstraZeneca. Alexandrina Balanean is a salaried employee of Cardinal Health, which received funding from Alexion, AstraZeneca Rare Disease for work performed on this study. Ajeet Gajra was a salaried employee of and owned stock in Cardinal Health (which received funding from Alexion, AstraZeneca Rare Disease for work performed on this study) at the time the study was conducted. Richard J. Nowak has received research support from Alexion, AstraZeneca Rare Disease, argenx, Genentech, Grifols, Immunovant, Inc., Momenta Pharmaceuticals, the Myasthenia Gravis Foundation of America, the National Institutes of Health (National Institute of Neurological Disorders and Stroke and National Institute of Allergy and Infectious Diseases), Ra Pharmaceuticals (now UCB), and Viela Bio Inc. (now Horizon Therapeutics); and consultancy fees from Alexion, AstraZeneca Rare Disease, argenx, Cour Pharmaceuticals, Grifols, Immunovant, Inc., Momenta Pharmaceuticals, Ra Pharmaceuticals (now UCB), Roivant Sciences, and Viela Bio (now Horizon Therapeutics). James F. Howard Jr. has received research support (paid to institution) from Ad Scientiam, Alexion, AstraZeneca Rare Disease, argenx, Cartesian Therapeutics, the Centers for Disease Control and Prevention (Atlanta, GA, USA), the Myasthenia Gravis Foundation of America, the Muscular Dystrophy Association, the National Institutes of Health (including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases), NMD Pharma, PCORI, and UCB Pharma; honoraria from Alexion AstraZeneca Rare Disease, argenx, Biohaven Ltd, Biologix Pharma, CheckRare CME, F. Hoffman-LaRoche, Horizon Therapeutics (now Amgen), Medscape CME, Merck EMD Serono, NMD Pharma, Novartis Pharma, PeerView CME, Physicians’ Education Resource (PER) CME, PlatformQ CME, Regeneron Pharmaceuticals, Sanofi US, UCB Pharma, and Zai Labs; and non-financial support from Alexion, AstraZeneca Rare Disease, argenx, Toleranzia AB, UCB Pharma, and Zai Labs.
Figures

References
-
- Melzer N, Ruck T, Fuhr P, Gold R, Hohlfeld R, Marx A, Melms A, Tackenberg B, Schalke B, Schneider-Gold C, Zimprich F, Meuth SG, Wiendl H (2016) Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol 263:1473–1494. 10.1007/s00415-016-8045-z 10.1007/s00415-016-8045-z - DOI - PMC - PubMed
-
- Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, Kuntz N, Massey JM, Melms A, Murai H, Nicolle M, Palace J, Richman DP, Verschuuren J, Narayanaswami P (2016) International consensus guidance for management of myasthenia gravis: executive summary. Neurology 87:419–425. 10.1212/wnl.0000000000002790 10.1212/wnl.0000000000002790 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

