Atezolizumab Before and After Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase II Nonrandomized Controlled Trial
- PMID: 39052256
- PMCID: PMC11273282
- DOI: 10.1001/jamaoncol.2024.1897
Atezolizumab Before and After Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase II Nonrandomized Controlled Trial
Abstract
Importance: Outcomes for patients with unresectable stage III non-small cell lung cancer (NSCLC) treated with chemoradiation therapy (CRT) have improved with adjuvant immune checkpoint inhibitors, with a reported 5-year overall survival benefit of approximately 10% for adjuvant durvalumab vs placebo after completion of CRT without progression and with preserved performance status. Starting atezolizumab prior to CRT may allow more patients to benefit from immunotherapy.
Objective: To evaluate clinical outcomes of patients treated with atezolizumab before and after CRT for unresectable stage III NSCLC.
Design, setting, and participants: This single-cohort, phase II, nonrandomized controlled trial was conducted at 11 US sites. Patients with pathologically confirmed, unresectable stage III NSCLC who were treatment naive and had good performance status were enrolled between January 3, 2018, and July 24, 2019. Data were locked on March 21, 2023.
Interventions: Patients received four 21-day cycles of atezolizumab, 1200 mg intravenously, with therapy administered on day 1 of each cycle. Patients not experiencing tumor progression continued to CRT (60 Gy to involved fields) concurrent with weekly carboplatin area under the curve of 2 and paclitaxel, 50 mg/m2, followed by planned consolidation carboplatin area under the curve of 6 and paclitaxel, 200 mg/m2, for two 21-day cycles. Patients not experiencing progression continued atezolizumab, 1200 mg, every 21 days to complete 1 year of therapy.
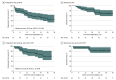
Main outcomes and measures: The primary end point was the disease control rate at 12 weeks. Secondary end points were progression-free survival, overall survival, overall response rate, safety, and translational science end points.
Results: A total of 62 patients (median [range] age, 63.9 [38.1-86.5] years; 32 female [51.6%]) were enrolled and received at least 1 dose of atezolizumab. The disease control rate at 12 weeks was 74.2% (80% CI, 65.7%-81.4%). Median progression-free survival was 30.0 months (95% CI, 15.8 to not evaluable), and the median overall survival was not reached. The overall survival rate at 24 months was 73.7% (95% CI, 63.4%-85.7%), and the overall response rate was 66.2%. Seventeen patients (27.4%) experienced grade 3 or higher immune-related adverse events, including 1 with grade 5 pneumonitis and 1 with grade 4 Guillain-Barré syndrome. Thirty patients (48.4%) experienced grade 3 or higher treatment-related adverse events.
Conclusions and relevance: These findings suggest that neoadjuvant atezolizumab merits further study based on safety and encouraging outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT03102242.
Conflict of interest statement
Figures


Comment in
-
Immunotherapy Before Chemoradiotherapy-More Is Better?JAMA Oncol. 2024 Sep 1;10(9):1219-1220. doi: 10.1001/jamaoncol.2024.0993. JAMA Oncol. 2024. PMID: 39052302 No abstract available.
References
-
- Vokes EE, Herndon JE II, Kelley MJ, et al. ; Cancer and Leukemia Group B . Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25(13):1698-1704. doi: 10.1200/JCO.2006.07.3569 - DOI - PubMed
-
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi: 10.1016/S1470-2045(14)71207-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

