Myeloid cells coordinately induce glioma cell-intrinsic and cell-extrinsic pathways for chemoresistance via GP130 signaling
- PMID: 39053460
- PMCID: PMC11384956
- DOI: 10.1016/j.xcrm.2024.101658
Myeloid cells coordinately induce glioma cell-intrinsic and cell-extrinsic pathways for chemoresistance via GP130 signaling
Abstract
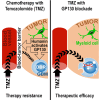
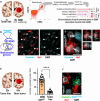
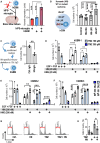
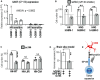
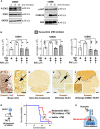
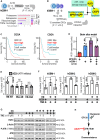
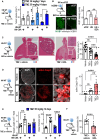
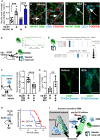
The DNA damage response (DDR) and the blood-tumor barrier (BTB) restrict chemotherapeutic success for primary brain tumors like glioblastomas (GBMs). Coherently, GBMs almost invariably relapse with fatal outcomes. Here, we show that the interaction of GBM and myeloid cells simultaneously induces chemoresistance on the genetic and vascular levels by activating GP130 receptor signaling, which can be addressed therapeutically. We provide data from transcriptomic and immunohistochemical screens with human brain material and pharmacological experiments with a humanized organotypic GBM model, proteomics, transcriptomics, and cell-based assays and report that nanomolar concentrations of the signaling peptide humanin promote temozolomide (TMZ) resistance through DDR activation. GBM mouse models recapitulating intratumoral humanin release show accelerated BTB formation. GP130 blockade attenuates both DDR activity and BTB formation, resulting in improved preclinical chemotherapeutic efficacy. Altogether, we describe an overarching mechanism for TMZ resistance and outline a translatable strategy with predictive markers to improve chemotherapy for GBMs.
Keywords: DDR; DNA damage response; GP130; IL6ST; TAM; blood-tumor barrier; chemotherapy; glioblastoma; humanin; temozolomide; tumor-associated myeloid cells.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Aasland D., Gotzinger L., Hauck L., Berte N., Meyer J., Effenberger M., Schneider S., Reuber E.E., Roos W.P., Tomicic M.T., et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR-CHK1, p21, and NF-κB. Cancer Res. 2019;79:99–113. doi: 10.1158/0008-5472.CAN-18-1733. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

