Diagnostic Accuracy of the Abbott BinaxNOW COVID-19 Antigen Card Test, Puerto Rico
- PMID: 39053895
- PMCID: PMC11300111
- DOI: 10.1111/irv.13305
Diagnostic Accuracy of the Abbott BinaxNOW COVID-19 Antigen Card Test, Puerto Rico
Abstract
Background: The COVID-19 pandemic underscored the need for rapid and accurate diagnostic tools. In August 2020, the Abbott BinaxNOW COVID-19 Antigen Card test became available as a timely and affordable alternative for SARS-CoV-2 molecular testing, but its performance may vary due to factors including timing and symptomatology. This study evaluates BinaxNOW diagnostic performance in diverse epidemiological contexts.
Methods: Using RT-PCR as reference, we assessed performance of the BinaxNOW COVID-19 test for SARS-CoV-2 detection in anterior nasal swabs from participants of two studies in Puerto Rico from December 2020 to May 2023. Test performance was assessed by days post symptom onset, collection strategy, vaccination status, symptomatology, repeated testing, and RT-PCR cycle threshold (Ct) values.
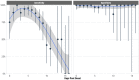
Results: BinaxNOW demonstrated an overall sensitivity of 84.1% and specificity of 98.8%. Sensitivity peaked within 1-6 days after symptom onset (93.2%) and was higher for symptomatic (86.3%) than asymptomatic (67.3%) participants. Sensitivity declined over the course of infection, dropping from 96.3% in the initial test to 48.4% in testing performed 7-14 days later. BinaxNOW showed 99.5% sensitivity in participants with low Ct values (≤ 25) but lower sensitivity (18.2%) for participants with higher Cts (36-40).
Conclusions: BinaxNOW demonstrated high sensitivity and specificity, particularly in early-stage infections and symptomatic participants. In situations where test sensitivity is crucial for clinical decision-making, nucleic acid amplification tests are preferred. These findings highlight the importance of considering clinical and epidemiological context when interpreting test results and emphasize the need for ongoing research to adapt testing strategies to emerging SARS-CoV-2 variants.
Keywords: Omicron; Puerto Rico; SARS‐CoV‐2; diagnostic accuracy; rapid antigen test; sensitivity.
© 2024 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
Sensitivity and Specificity of SARS-CoV-2 Rapid Antigen Detection Tests Using Oral, Anterior Nasal, and Nasopharyngeal Swabs: a Diagnostic Accuracy Study.Microbiol Spectr. 2022 Feb 23;10(1):e0202921. doi: 10.1128/spectrum.02029-21. Epub 2022 Feb 2. Microbiol Spectr. 2022. PMID: 35107327 Free PMC article.
-
Performance and Implementation Evaluation of the Abbott BinaxNOW Rapid Antigen Test in a High-Throughput Drive-Through Community Testing Site in Massachusetts.J Clin Microbiol. 2021 Apr 20;59(5):e00083-21. doi: 10.1128/JCM.00083-21. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33622768 Free PMC article.
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
-
Comparison of diagnostic accuracy of rapid antigen tests for COVID-19 compared to the viral genetic test in adults: a systematic review and meta-analysis.JBI Evid Synth. 2024 Oct 1;22(10):1939-2002. doi: 10.11124/JBIES-23-00291. JBI Evid Synth. 2024. PMID: 39188132 Free PMC article.
Cited by
-
Longitudinal analysis of SARS-CoV-2 IgG antibody durability in Puerto Rico.Sci Rep. 2024 Dec 28;14(1):30743. doi: 10.1038/s41598-024-80465-4. Sci Rep. 2024. PMID: 39730470 Free PMC article.
References
-
- World Health Organization , “WHO Coronavirus (COVID‐19) Dashboard,” (2023), accessed October 12, 2023, https://covid19.who.int/.
-
- Centers for Disease Control and Prevention , “Isolation and Precautions for People With COVID‐19,” (2023), accessed October 3, 2023, https://www.cdc.gov/coronavirus/2019‐ncov/your‐health/isolation.html.
-
- Wong J. M., Volkman H. R., Adams L. E., et al., “Clinical Features of COVID‐19, Dengue, and Influenza Among Adults Presenting to Emergency Departments and Urgent Care Clinics‐Puerto Rico, 2012‐2021,” The American Journal of Tropical Medicine and Hygiene 108, no. 1 (2023): 107–114, 10.4269/ajtmh.22-0149. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

