Development and application of a qualitative rapid analysis framework in a hybrid trial within primary care
- PMID: 39053958
- PMCID: PMC11284935
- DOI: 10.1136/bmjopen-2023-076792
Development and application of a qualitative rapid analysis framework in a hybrid trial within primary care
Abstract
Context: In the context of iterative feedback loops to support real-time policy decision making, and an emphasis on speeding up adoption of evidence-based interventions, qualitative healthcare researchers are increasingly expected to produce rapid results and products. Traditional qualitative methods have been adapted for this purpose.
Objective: To develop and apply a rapid analysis framework in a process evaluation for the VICTORION-Spirit study; a ground-breaking hybrid trial examining real-world delivery of inclisiran-a cholesterol-lowering treatment-in primary care.
Design: We developed a rapid analysis framework, using a summary template, to analyse data from semistructured telephone interviews.
Setting: Primary care in Greater Manchester, UK.
Participants: Patients who had received inclisiran as part of the VICTORION-Spirit trial (56), providers delivering inclisiran (28) and representatives from the Academic Health Science Network (8) participated in the original study.
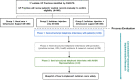
Results: The rapid analysis framework we developed and applied comprised six steps: (1) creating a summary template based on the five Consolidated Framework for Implementation Research domains; (2) test-driving, refining and finalising the summary template; (3) completing the template soon after each interview using field notes; (4) discussing analysis as a team; (5) transferring summaries to a matrix; and (6) using the summary matrix to inform presentations and interim reports for stakeholders. Our rapid analysis framework saved time and improved efficiency, as we were able to feedback barriers to stakeholders in real time via presentations.
Conclusions: Rapid analysis in applied healthcare research can produce timely and trustworthy findings. Our rapid analysis framework would be useful within studies where there is a need to feedback to stakeholders and adjust implementation strategies accordingly in real time. Thus, supporting successful implementation efforts and accelerating adoption.
Trial registration number: NCT04807400, 19/03/2021.
Keywords: Clinical Trial; Implementation Science; Patient Participation; Primary Care; QUALITATIVE RESEARCH; STATISTICS & RESEARCH METHODS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Beebe J. Rapid qualitative inquiry: a field guide to team-based assessment. Rowman & Littlefield; 2014. p. 283.
-
- Vindrola-Padros C. Rapid ethnographies: a practical guide. Cambridge University Press; 2021. https://www.cambridge.org/gb/academic/subjects/social-science-research-m... Available.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical