Selective early medical treatment of the patent ductus arteriosus in extremely low gestational age infants: a pilot randomised controlled trial protocol (SMART-PDA)
- PMID: 39053961
- PMCID: PMC11284877
- DOI: 10.1136/bmjopen-2024-087998
Selective early medical treatment of the patent ductus arteriosus in extremely low gestational age infants: a pilot randomised controlled trial protocol (SMART-PDA)
Abstract
Introduction: Patent ductus arteriosus (PDA) is the most common cardiovascular problem that develops in extremely preterm infants and is associated with poor clinical outcomes. Uncertainty exists on whether early pharmacotherapeutic treatment of a clinically symptomatic and echocardiography-confirmed haemodynamically significant PDA in extremely preterm infants improves outcomes. Given the wide variation in the approach to PDA treatment in this gestational age (GA) group, a randomised trial design is essential to address the question. Before embarking on a large RCT in this vulnerable population, it is important to establish the feasibility of such a trial.
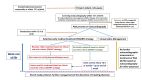
Methods and analysis: Design: a multi-centre, open-labelled, parallel-designed pilot randomised controlled trial. Participants: preterm infants born <26 weeks of gestation with a PDA diagnosed within 72 hours after birth. Intervention (selective early medical treatment (SMART) strategy): selective early pharmacological treatment of a moderate-severe PDA shunt (identified based on pre-defined clinical signs and routine screening echocardiography) within the first 72 postnatal hours with provision for repeat treatment if moderate-severe shunt persists. Comparison (early conservative management strategy): no treatment of PDA in the first postnatal week. Primary outcomes: (1) proportion of eligible infants recruited during the study period; (2) proportion of randomised infants treated outside of protocol-mandated therapy. Sites and sample size: the study is being conducted in seven neonatal intensive care units across Canada and the USA with a target of 100 randomised infants. Analysis: the primary feasibility outcomes will be expressed as proportions. A pre-planned Bayesian analysis will be conducted for secondary clinical outcomes such as mortality, severe intraventricular haemorrhage, procedural PDA closure and chronic lung disease to aid stakeholders including parent representatives decide on the appropriateness of enrolling this vulnerable population in a larger trial if the feasibility of recruitment in the pilot trial is established.
Ethics and dissemination: The study has been approved by the IWK Research Ethics Board (#1027298) and six additional participating sites. On the completion of the study, results will be presented at national and international meetings, published in peer-reviewed journals and incorporated into existing systematic reviews.
Trial registration number: NCT05011149 (WHO Trial Registration Data Set in Appendix A).
Protocol version: Ver 7.2 (dated July 19, 2023).
Keywords: NEONATOLOGY; Neonatal intensive & critical care; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Chung MY, Fang PC, Chung CH, et al. Risk factors for hemodynamically-unrelated cystic periventricular leukomalacia in very low birth weight premature infants. J Formos Med Assoc Taiwan Yi Zhi. 2005;104:571–7. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical