The historical background of hereditary cystatin C amyloid angiopathy: Genealogical, pathological, and clinical manifestations
- PMID: 39054254
- PMCID: PMC11835439
- DOI: 10.1111/bpa.13291
The historical background of hereditary cystatin C amyloid angiopathy: Genealogical, pathological, and clinical manifestations
Abstract
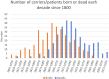
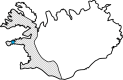
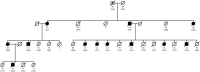
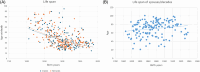
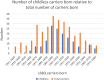
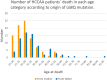
Hereditary cystatin C amyloid angiopathy (HCCAA) is an Icelandic disease that belongs to a disease class called cerebral amyloid angiopathy, a group of heterogenous diseases presenting with aggregation of amyloid complexes and deposition predominantly in the central nervous system. HCCAA is dominantly inherited, caused by L68Q mutation in the cystatin C gene, leading to aggregation of the cystatin C protein. HCCAA is a very progressive and severe disease, with widespread cerebral and parenchymal cystatin C and collagen IV deposition within the central nervous system (CNS) but also in other organs in the body, for example, in the skin. Most L68Q carriers have clinical symptoms characterized by recurrent hemorrhages and dementia, between the age of 20-30 years. If the carriers survive the first hemorrhage, the frequency and severity of the hemorrhages tend to increase, resulting in death at average of 30 years with mean number of major hemorrhages ranging from 3.2 to 3.9 over a 5-year average life span. The pathogenesis of the disease in carriers is very similar in the CNS and in the skin based on autopsy studies, thus skin biopsies can be used to monitor the progression of the disease by quantifying the cystatin C immunoreactivity. The cystatin C deposition always colocalizes with collagen IV and fibroblasts in the skin are found to be the main cell type responsible for the deposition of both proteins. No therapy is available for this devastating disease.
Keywords: N‐acetylcysteine; cerebral amyloid angiopathy; collagen IV; cystatin C; hemorrhage; hereditary cystatin C amyloid angiopathy.
© 2024 The Author(s). Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Figures



References
-
- Arnason A. Apoplexie and ihre vererbung. Acta Pcyshiat Neurol. 1935;7:1–180.
-
- Yamada M, Naiki H. Cerebral amyloid angiopathy. Prog Mol Biol Transl Sci. 2012;107:41–78. - PubMed
-
- Gudmundsson G, Hallgrimsson J, Jonasson TA, Bjarnason O. Hereditary cerebral haemorrhage with amyloidosis. Brain. 1972;95(2):387–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

