Oxytocin induces the formation of distinctive cortical representations and cognitions biased toward familiar mice
- PMID: 39054324
- PMCID: PMC11272796
- DOI: 10.1038/s41467-024-50113-6
Oxytocin induces the formation of distinctive cortical representations and cognitions biased toward familiar mice
Abstract
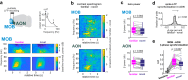
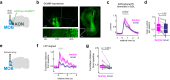
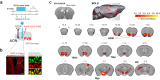
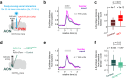
Social recognition is essential for the formation of social structures. Many times, recognition comes with lesser exploration of familiar animals. This lesser exploration has led to the assumption that recognition may be a habituation memory. The underlying memory mechanisms and the thereby acquired cortical representations of familiar mice have remained largely unknown, however. Here, we introduce an approach directly examining the recognition process from volatile body odors among male mice. We show that volatile body odors emitted by mice are sufficient to identify individuals and that more salience is assigned to familiar mice. Familiarity is encoded by reinforced population responses in two olfactory cortex hubs and communicated to other brain regions. The underlying oxytocin-induced plasticity promotes the separation of the cortical representations of familiar from other mice. In summary, neuronal encoding of familiar animals is distinct and utilizes the cortical representational space more broadly, promoting storage of complex social relationships.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Thor, D. H. & Holloway, W. R. Social Memory of the Male Laboratory Rat. J. Comp. Physiol. Psychol.96, 1000–1006 (1982).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

