IgG replacement in multiple myeloma
- PMID: 39054331
- PMCID: PMC11272770
- DOI: 10.1038/s41408-024-01107-6
IgG replacement in multiple myeloma
Abstract
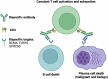
T cell engagers (TCE) such as chimeric antigen receptor (CAR) T cell therapy and bispecific antibodies (BiAbs) for the treatment of multiple myeloma (MM) have significantly improved clinical outcomes, but have also raised awareness for ensuing post-treatment secondary immunodeficiency and hypogammaglobulinemia (HG). As patients with MM live longer, recurrent infections become a significant component of therapy-associated morbidity and mortality. Treatment of HG with immunoglobulin G replacement therapy (IgG-RT) has been a mainstay of the primary immunodeficiency (PI) world, and extrapolation to MM has recently started to show promising clinical outcomes. However, IgG-RT initiation, dosing, route, timing, monitoring, and management in MM has not been standardized in the setting of TCE. Progress in MM treatment will involve greater recognition and screening of underlying secondary immunodeficiency, identification of risk-stratification markers, optimizing IgG-RT management, and implementing other approaches to decrease the risk of infection. In this review, we summarize infection risk, risk of HG, and management strategies for IgG-RT in patients with relapsed MM after TCE.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023;41:1265–74. 10.1200/JCO.22.00842 - DOI - PMC - PubMed
-
- Otani IM, Lehman HK, Jongco AM, Tsao LR, Azar AE, Tarrant TK, et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: a Work Group Report of the AAAAI Primary Immunodeficiency and Altered Immune Response Committees. J Allergy Clin Immunol. 2022;149:1525–60. 10.1016/j.jaci.2022.01.025 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

