Non-invasive mapping of brown adipose tissue activity with magnetic resonance imaging
- PMID: 39054361
- PMCID: PMC11272596
- DOI: 10.1038/s42255-024-01082-z
Non-invasive mapping of brown adipose tissue activity with magnetic resonance imaging
Abstract
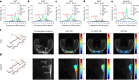
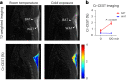
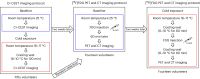
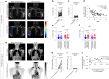
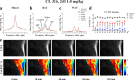
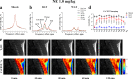
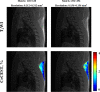
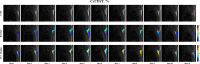
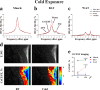
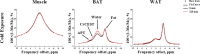
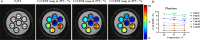
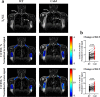
Thermogenic brown adipose tissue (BAT) has a positive impact on whole-body metabolism. However, in vivo mapping of BAT activity typically relies on techniques involving ionizing radiation, such as [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET) and computed tomography (CT). Here we report a noninvasive metabolic magnetic resonance imaging (MRI) approach based on creatine chemical exchange saturation transfer (Cr-CEST) contrast to assess in vivo BAT activity in rodents and humans. In male rats, a single dose of the β3-adrenoceptor agonist (CL 316,243) or norepinephrine, as well as cold exposure, triggered a robust elevation of the Cr-CEST MRI signal, which was consistent with the [18F]FDG PET and CT data and 1H nuclear magnetic resonance measurements of creatine concentration in BAT. We further show that Cr-CEST MRI detects cold-stimulated BAT activation in humans (both males and females) using a 3T clinical scanner, with data-matching results from [18F]FDG PET and CT measurements. This study establishes Cr-CEST MRI as a promising noninvasive and radiation-free approach for in vivo mapping of BAT activity.
© 2024. The Author(s).
Conflict of interest statement
K.C., Z.C. and Z.L. filed a US patent application through the University of Illinois at Chicago, ‘Systems and methods for magnetic resonance imaging (MRI) noninvasive mapping of brown adipose tissue activity’. The other authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- R01 CA283548/CA/NCI NIH HHS/United States
- U21A6005/National Natural Science Foundation of China (National Science Foundation of China)
- R01 DK135722/DK/NIDDK NIH HHS/United States
- R01DK135722/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01CA283548/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
LinkOut - more resources
Full Text Sources
Medical

