Antiplasmodial potential of isolated xanthones from Mesua ferrea Linn. roots: an in vitro and in silico molecular docking and pharmacokinetics study
- PMID: 39054443
- PMCID: PMC11270968
- DOI: 10.1186/s12906-024-04580-5
Antiplasmodial potential of isolated xanthones from Mesua ferrea Linn. roots: an in vitro and in silico molecular docking and pharmacokinetics study
Abstract
Background: Malaria is a major global health concern, particularly in tropical and subtropical countries. With growing resistance to first-line treatment with artemisinin, there is an urgent need to discover novel antimalarial drugs. Mesua ferrea Linn., a plant used in traditional medicine for various purposes, has previously been investigated by our research group for its cytotoxic properties. The objective of this study was to explore the compounds isolated from M. ferrea with regards to their potential antiplasmodial activity, their interaction with Plasmodium falciparum lactate dehydrogenase (PfLDH), a crucial enzyme for parasite survival, and their pharmacokinetic and toxicity profiles.
Methods: The isolated compounds were assessed for in vitro antiplasmodial activity against a multidrug-resistant strain of P. falciparum K1 using a parasite lactate dehydrogenase (pLDH) assay. In vitro cytotoxicity against Vero cells was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The interactions between the isolated compounds and the target enzyme PfLDH were investigated using molecular docking. Additionally, pharmacokinetic and toxicity properties were estimated using online web tools SwissADME and ProTox-II, respectively.
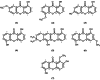
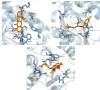
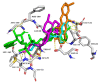
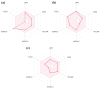
Results: Among the seven compounds isolated from M. ferrea roots, rheediachromenoxanthone (5), which belongs to the pyranoxanthone class, demonstrated good in vitro antiplasmodial activity, with the IC50 being 19.93 µM. Additionally, there was no toxicity towards Vero cells (CC50 = 112.34 µM) and a selectivity index (SI) of 5.64. Molecular docking analysis revealed that compound (5) exhibited a strong binding affinity of - 8.6 kcal/mol towards PfLDH and was stabilized by forming hydrogen bonds with key amino acid residues, including ASP53, TYR85, and GLU122. Pharmacokinetic predictions indicated that compound (5) possessed favorable drug-like properties and desired pharmacokinetic characteristics. These include high absorption in the gastrointestinal tract, classification as a non-substrate of permeability glycoprotein (P-gp), non-inhibition of CYP2C19, ease of synthesis, a high predicted LD50 value of 4,000 mg/kg, and importantly, non-hepatotoxic, non-carcinogenic, and non-cytotoxic effects.
Conclusions: This study demonstrated that compounds isolated from M. ferrea exhibit activity against P. falciparum. Rheediachromenoxanthone has significant potential as a scaffold for the development of potent antimalarial drugs.
Keywords: Mesua ferrea Linn.; Plasmodium falciparum; Antiplasmodial activity; In silico pharmacokinetics; Molecular docking; Xanthones.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- WHO. World malaria report 2022. Geneva: World Health Organization; 2022.
-
- Bennett A, Bisanzio D, Yukich JO, Mappin B, Fergus CA, Lynch M, Cibulskis RE, Bhatt S, Weiss DJ, Cameron E, et al. Population coverage of artemisinin-based combination treatment in children younger than 5 years with fever and Plasmodium falciparum infection in Africa, 2003–2015: a modelling study using data from national surveys. Lancet Glob Health. 2017;5(4):e418–27. - PMC - PubMed
-
- Tindana P, Guissou R, Bolarinwa OA, Tou F, de Haan F, Dhorda M, Dondorp AM, Amaratunga C, Mokuolu OA, Ouedraogo JB, et al. Ethical considerations in deploying triple artemisinin-based combination therapies for malaria: an analysis of stakeholders’ perspectives in Burkina Faso and Nigeria. PLoS ONE. 2022;17(9):e0273249. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

