Auranofin inhibition of thioredoxin reductase sensitizes lung neuroendocrine tumor cells (NETs) and small cell lung cancer (SCLC) cells to sorafenib as well as inhibiting SCLC xenograft growth
- PMID: 39054566
- PMCID: PMC11275529
- DOI: 10.1080/15384047.2024.2382524
Auranofin inhibition of thioredoxin reductase sensitizes lung neuroendocrine tumor cells (NETs) and small cell lung cancer (SCLC) cells to sorafenib as well as inhibiting SCLC xenograft growth
Abstract
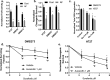
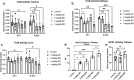
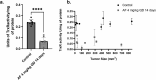
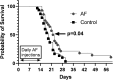
Thioredoxin Reductase (TrxR) functions to recycle thioredoxin (Trx) during hydroperoxide metabolism mediated by peroxiredoxins and is currently being targeted using the FDA-approved anti-rheumatic drug, auranofin (AF), to selectively sensitize cancer cells to therapy. AF treatment decreased TrxR activity and clonogenic survival in small cell lung cancer (SCLC) cell lines (DMS273 and DMS53) as well as the H727 atypical lung carcinoid cell line. AF treatment also significantly sensitized DMS273 and H727 cell lines in vitro to sorafenib, an FDA-approved multi-kinase inhibitor that depleted intracellular glutathione (GSH). The pharmacokinetic, pharmacodynamic, and safety profile of AF was examined in nude mice with DMS273 xenografts administered AF intraperitoneally at 2 mg/kg or 4 mg/kg (IP) once (QD) or twice daily (BID) for 1-5 d. Plasma levels of AF were 10-20 μM (determined by mass spectrometry of gold), and the optimal inhibition of TrxR activity was obtained at 4 mg/kg once daily, with no effect on glutathione peroxidase 1 activity. This AF treatment extended for 14 d, inhibited TrxR (>75%), and resulted in a significant prolongation of median overall survival from 19 to 23 d (p = .04, N = 30 controls, 28 AF). In this experiment, there were no observed changes in animal bodyweight, complete blood counts (CBCs), bone marrow toxicity, blood urea nitrogen, or creatinine. These results support the hypothesis that AF effectively inhibits TrxR both in vitro and in vivo in SCLC, sensitizes NETs and SCLC to sorafenib, and could be repurposed as an adjuvant therapy with targeted agents that induce disruptions in thiol metabolism.
Keywords: auranofin; dms273; glutathione; h727; lung neuroendocrine tumor; small cell lung cancer; sorafenib; thioredoxin reductase.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Update of
-
Auranofin Inhibition of Thioredoxin Reductase Sensitizes Lung Neuroendocrine Tumor Cells (NETs) and Small Cell Lung Cancer (SCLC) Cells to Sorafenib as well as Inhibiting SCLC Xenograft Growth.bioRxiv [Preprint]. 2024 Jan 30:2023.05.07.539772. doi: 10.1101/2023.05.07.539772. bioRxiv. 2024. Update in: Cancer Biol Ther. 2024 Dec 31;25(1):2382524. doi: 10.1080/15384047.2024.2382524. PMID: 37215042 Free PMC article. Updated. Preprint.
References
-
- Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, Nabet BY, Fujimoto J, Solis LM, Lu W, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. [2021 Mar 8]. 39(3):346–60 e7. doi:10.1016/j.ccell.2020.12.014. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
