Prenatal assessment of brain malformations on neuroimaging: an expert panel review
- PMID: 39054600
- PMCID: PMC11730443
- DOI: 10.1093/brain/awae253
Prenatal assessment of brain malformations on neuroimaging: an expert panel review
Abstract
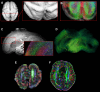
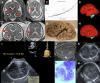
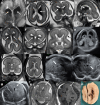
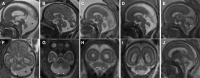
Brain malformations represent a heterogeneous group of abnormalities of neural morphogenesis, often associated with aberrations of neuronal connectivity and brain volume. Prenatal detection of brain malformations requires a clear understanding of embryology and developmental morphology through the various stages of gestation. This expert panel review is written with the central aim of providing an easy-to-understand road map to improve prenatal detection and characterization of structural malformations based on the current understanding of normal and aberrant brain development. For every developmental stage, the utility of each available neuroimaging modality, including prenatal multiplanar neuro sonography, anatomical MRI and advanced MRI techniques, as well as further insights from post-mortem imaging, has been highlighted.
Keywords: brain; fetal MRI; fetal ultrasound; malformations of cortical development; neuroimaging.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Raybaud C, Widjaja E. Development and dysgenesis of the cerebral cortex: Malformations of cortical development. Neuroimaging Clin N Am. 2011;21:483–543. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

