TM6SF2 E167K variant decreases PNPLA3-mediated PUFA transfer to promote hepatic steatosis and injury in MASLD
- PMID: 39054606
- PMCID: PMC11540376
- DOI: 10.3350/cmh.2024.0268
TM6SF2 E167K variant decreases PNPLA3-mediated PUFA transfer to promote hepatic steatosis and injury in MASLD
Abstract
Backgrounds/aims: Transmembrane 6 superfamily member 2 (TM6SF2) E167K variant is closely associated with the occurrence and development of metabolic dysfunction-associated steatotic liver disease (MASLD). However, the role and mechanism of TM6SF2 E167K variant during MASLD progression are not yet fully understood.
Methods: The Tm6sf2167K knock-in (KI) mice were subjected to high-fat diet (HFD). Hepatic lipid levels of Tm6sf2167K KI mice were detected by lipidomics analysis. Thin-layer chromatography (TLC) was used to measure the newly synthesized triglyceride (TG) and phosphatidylcholine (PC).
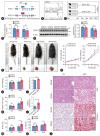
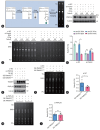
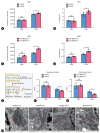
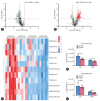
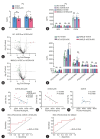
Results: The TM6SF2 E167K variant significantly aggravated hepatic steatosis and injury in HFD-induced mice. Decreased polyunsaturated PC level and increased polyunsaturated TG level were found in liver tissue of HFD-induced Tm6sf2167K KI mice. Mechanistic studies demonstrated that the TM6SF2 E167K variant increased the interaction between TM6SF2 and PNPLA3, and impaired PNPLA3-mediated transfer of polyunsaturated fatty acids (PUFAs) from TG to PC. The TM6SF2 E167K variant increased the level of fatty acid-induced malondialdehyde and reactive oxygen species, and decreased fatty acid-downregulated cell membrane fluidity. Additionally, the TM6SF2 E167K variant decreased the level of hepatic PC containing C18:3, and dietary supplementation of PC containing C18:3 significantly attenuated the TM6SF2 E167K-induced hepatic steatosis and injury in HFD-fed mice.
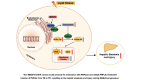
Conclusion: The TM6SF2 E167K variant could promote its interaction with PNPLA3 and inhibit PNPLA3-mediated transfer of PUFAs from TG to PC, resulting in the hepatic steatosis and injury during MASLD progression. PC containing C18:3 could act as a potential therapeutic supplement for MASLD patients carrying the TM6SF2 E167K variant.
Keywords: Hepatic steatosis; Metabolic dysfunction-associated steatotic liver disease; Patatin-like phospholipase domain-containing protein 3; Polyunsaturated fatty acids; Transmembrane 6 superfamily member 2.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures



References
-
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542–1556. - PubMed
-
- Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: A review. JAMA. 2020;323:1175–1183. - PubMed
-
- Le MH, Le DM, Baez TC, Wu Y, Ito T, Lee EY, et al. Global incidence of non-alcoholic fatty liver disease: A systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol. 2023;79:287–295. - PubMed
-
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. - PubMed
-
- Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–324. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

