Synthesis of alcohols: streamlined C1 to C n hydroxyalkylation through photoredox catalysis
- PMID: 39055000
- PMCID: PMC11268494
- DOI: 10.1039/d4sc02696a
Synthesis of alcohols: streamlined C1 to C n hydroxyalkylation through photoredox catalysis
Abstract
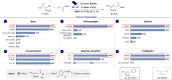
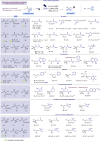
Naturally occurring and readily available α-hydroxy carboxylic acids (AHAs) are utilized as platforms for visible light-mediated oxidative CO2-extrusion furnishing α-hydroxy radicals proved to be versatile C1 to Cn hydroxyalkylating agents. The direct decarboxylative Giese reaction (DDGR) is operationally simple, not requiring activator or sacrificial oxidants, and enables the synthesis of a diverse range of hydroxylated products, introducing connectivity typically precluded from conventional polar domains. Notably, the methodology has been extended to widely used glycolic acid resulting in a highly efficient and unprecedented C1 hydroxyhomologation tactic. The use of flow technology further facilitates scalability and adds green credentials to this synthetic methodology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Continuous Flow Decarboxylative Monofluoroalkylation Enabled by Photoredox Catalysis.JACS Au. 2025 Feb 2;5(2):684-692. doi: 10.1021/jacsau.4c00902. eCollection 2025 Feb 24. JACS Au. 2025. PMID: 40017745 Free PMC article.
-
Visible-Light Photoredox-Catalyzed Giese Reaction: Decarboxylative Addition of Amino Acid Derived α-Amino Radicals to Electron-Deficient Olefins.Chemistry. 2016 Sep 12;22(38):13464-8. doi: 10.1002/chem.201602257. Epub 2016 Jun 20. Chemistry. 2016. PMID: 27321136
-
Decarboxylative Fluorination of Aliphatic Carboxylic Acids via Photoredox Catalysis.J Am Chem Soc. 2015 May 6;137(17):5654-7. doi: 10.1021/jacs.5b02244. Epub 2015 Apr 27. J Am Chem Soc. 2015. PMID: 25881929 Free PMC article.
-
Alkoxy Radicals See the Light: New Paradigms of Photochemical Synthesis.Chem Rev. 2022 Jan 26;122(2):2429-2486. doi: 10.1021/acs.chemrev.1c00256. Epub 2021 Oct 6. Chem Rev. 2022. PMID: 34613698 Review.
-
Generation of alkyl and acyl radicals by visible-light photoredox catalysis: direct activation of C-O bonds in organic transformations.Beilstein J Org Chem. 2024 Jun 14;20:1348-1375. doi: 10.3762/bjoc.20.119. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38887583 Free PMC article. Review.
Cited by
-
Continuous Flow Decarboxylative Monofluoroalkylation Enabled by Photoredox Catalysis.JACS Au. 2025 Feb 2;5(2):684-692. doi: 10.1021/jacsau.4c00902. eCollection 2025 Feb 24. JACS Au. 2025. PMID: 40017745 Free PMC article.
References
LinkOut - more resources
Full Text Sources

