Ambipolar conjugated ladder polymers by room-temperature Knoevenagel polymerization
- PMID: 39055013
- PMCID: PMC11268504
- DOI: 10.1039/d4sc03222e
Ambipolar conjugated ladder polymers by room-temperature Knoevenagel polymerization
Abstract
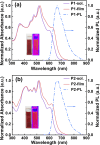
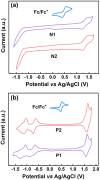
Two soluble conjugated ladder polymers (cLPs), decorated with multiple electron-poor species (i.e., cyano groups, fused pentagons, and N-heterocyclic rings), have been synthesized from the newly developed tetraketo-functionalized double aza[5]helicene building blocks using a single-step Knoevenagel polycondensation strategy. This facile approach features mild conditions (e.g., room temperature) and high efficiency, allowing us to quickly access a nonalternant ladder-like conjugated system with the in situ formation of multicyano substituents in the backbone. Analysis by 1H NMR, FT-Raman, and FT-IR spectra confirms the successful synthesis of the resulting cLPs. The combination of theoretical calculations and experimental characterizations reveals that the slightly contorted geometry coupled with a random assignment of trans- and cis-isomeric repeating units in each main chain contributes to improving the solubility of such rigid, multicyano nanoribbon systems. Apart from outstanding thermal stability, the resulting cLPs exhibit attractive red fluorescence, excellent redox properties, and strong π-π interactions coupled with orderly face-on packing in their thin-film states. They are proven to be the first example of ambipolar cLPs that show satisfactory hole and electron mobilities of up to 0.01 and 0.01 cm2 V-1 s-1, respectively. As we demonstrate, the Knoevenagel polycondensation chemistries open a new window to create complex and unique ladder-like nanoribbon systems under mild reaction conditions that are otherwise challenging to achieve.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Yang J. S. J. Fang L. Chem. 2024;10:1–57.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

