Tetraborylation of p-Benzynes Generated by the Masamune-Bergman Cyclization through Reaction Design Based on the Reaction Path Network
- PMID: 39055142
- PMCID: PMC11267532
- DOI: 10.1021/jacsau.4c00302
Tetraborylation of p-Benzynes Generated by the Masamune-Bergman Cyclization through Reaction Design Based on the Reaction Path Network
Abstract
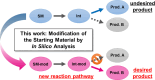
Designing the reactant molecule of an initial reaction, based on quantum chemical pathway exploration, enabled us to access a new reaction, i.e., the tetraborylation reaction of p-benzynes generated from 1,2-diethynylbenzene derivatives, using bis(pinacolato)diborane(4) (B2pin2). Based on the reaction path network generated via the artificial-force-induced reaction (AFIR) method, desired and undesired paths were identified and used to modify the chemical structure of the reactant. After the in silico screening, the optimal structure of the reactant was determined to be a 1,2-diethynylbenzene derivative with a butylene linker. The reaction of the optimized reactant and its derivatives with an excess of B2pin2 gave the tetraborylated products in good yields (up to 58%). It is quite intriguing that the two carbons of p-benzyne behave formally as dicarbenes in this reaction.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
