Engineering Bifunctional Galactokinase/Uridyltransferase Chimera for Enhanced UDP-d-Xylose Production
- PMID: 39055162
- PMCID: PMC11267548
- DOI: 10.1021/jacsau.4c00288
Engineering Bifunctional Galactokinase/Uridyltransferase Chimera for Enhanced UDP-d-Xylose Production
Abstract
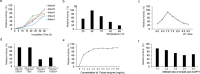
The biotechnological production of uridine diphosphate-d-xylose (UDP-d-xylose), the glycosyl donor in enzymatic for d-xylose, is an important precursor for advancing glycoengineering research on biopharmaceuticals such as heparin and glycosaminoglycans. Leveraging a recently discovered UDP-xylose salvage pathway, we have engineered a series of bifunctional chimeric biocatalysts derived from Solitalea canadensis galactokinase/uridyltransferase, facilitating the conversion of d-xylose to UDP-d-xylose. This study elucidates the novel assembly of eight fusion protein constructs, differing in domain orientations and linker peptide lengths, to investigate their functional expression in Escherichia coli, resulting in the synthesis of the first bifunctional enzyme that orchestrates a direct transformation from d-xylose to UDP-d-xylose. Fusion constructs with a NH2-GSGGGSGHM-COOH peptide linker demonstrated the highest expression and catalytic tenacity. For the highest catalytic conversion from d-xylose to UDP-d-xylose, we established an optimum pH of 7.0 and a temperature optimum of 30 °C, with an optimal fusion enzyme concentration of 3.3 mg/mL for large-scale UDP-d-xylose production. Insights into ATP and ADP inhibition further helped to optimize the reaction conditions. Testing various ratios of unfused galactokinase and uridyltransferase biocatalysts for UDP-xylose synthesis from d-xylose revealed that a 1:1 ratio was optimal. The K cat/K m value for the NH2-GSGGGSGHM-COOH peptide linker showed a 10% improvement compared with the unfused counterparts. The strategic design of these fusion enzymes efficiently routes for the convenient and efficient biocatalytic synthesis of xylosides in biotechnological and pharmaceutical applications.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
