Baeyer-Villiger oxidation: a promising tool for the synthesis of natural products: a review
- PMID: 39055269
- PMCID: PMC11270005
- DOI: 10.1039/d4ra03914a
Baeyer-Villiger oxidation: a promising tool for the synthesis of natural products: a review
Abstract
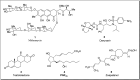
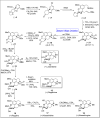
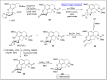
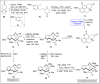
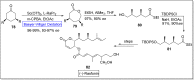
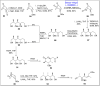
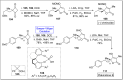
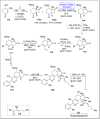
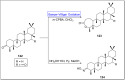
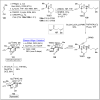
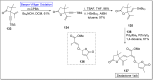
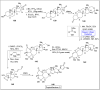
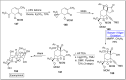
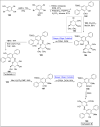
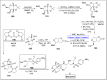
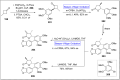
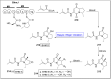
Baeyer-Villiger oxidation is a well-known reaction utilized for the synthesis of lactones and ester functionalities from ketones. Chiral lactones can be synthesized from chiral or racemic ketones by employing asymmetric Baeyer-Villiger oxidation. These lactones act as key intermediates in the synthesis of most of the biologically active natural products, their analogues, and derivatives. Various monooxygenases and oxidizing agents facilitate BV oxidation, providing a broad range of synthetic applications in organic chemistry. The variety of enzymatic and chemoselective Baeyer-Villiger oxidations and their substantial role in the synthesis of natural products i.e., alkaloids, polyketides, fatty acids, terpenoids, etc. (reported since 2018) have been summarized in this review article.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










Similar articles
-
[Baeyer-Villiger monooxygenases in the biosynthesis of microbial secondary metabolites].Sheng Wu Gong Cheng Xue Bao. 2019 Mar 25;35(3):351-362. doi: 10.13345/j.cjb.180294. Sheng Wu Gong Cheng Xue Bao. 2019. PMID: 30912344 Review. Chinese.
-
Discovery of Two Native Baeyer-Villiger Monooxygenases for Asymmetric Synthesis of Bulky Chiral Sulfoxides.Appl Environ Microbiol. 2018 Jul 2;84(14):e00638-18. doi: 10.1128/AEM.00638-18. Print 2018 Jul 15. Appl Environ Microbiol. 2018. PMID: 29752270 Free PMC article.
-
Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations.Nature. 2001 Jul 26;412(6845):423-5. doi: 10.1038/35086546. Nature. 2001. PMID: 11473313
-
Efficient Synthesis of Methyl 3-Acetoxypropionate by a Newly Identified Baeyer-Villiger Monooxygenase.Appl Environ Microbiol. 2019 May 16;85(11):e00239-19. doi: 10.1128/AEM.00239-19. Print 2019 Jun 1. Appl Environ Microbiol. 2019. PMID: 30926727 Free PMC article.
-
Towards large-scale synthetic applications of Baeyer-Villiger monooxygenases.Trends Biotechnol. 2003 Jul;21(7):318-23. doi: 10.1016/S0167-7799(03)00144-6. Trends Biotechnol. 2003. PMID: 12837617 Review.
Cited by
-
Biocatalytic production of 3-hydroxypropionic acid precursors using a regioselective Baeyer-Villiger monooxygenase.Sci Rep. 2025 Apr 22;15(1):13986. doi: 10.1038/s41598-025-96783-0. Sci Rep. 2025. PMID: 40263398 Free PMC article.
-
Oxone®-mediated Dakin-like reaction to synthesize hydroxyarenes: an approach using pyrazolo[1,5-a]pyrimidines.RSC Adv. 2025 Jul 7;15(29):23441-23447. doi: 10.1039/d5ra02812d. eCollection 2025 Jul 4. RSC Adv. 2025. PMID: 40626061 Free PMC article.
References
-
- Cavarzan A. Bianchini G. Sgarbossa P. Lefort L. Gladiali S. Scarso A. Strukul G. Chem.–Eur. J. 2009;15:7930–7939. - PubMed
-
- Leisch H. Morley K. Lau P. C. Chem. Rev. 2011;111:4165–4222. - PubMed
-
- Jiménez-Sanchidrián C. Ruiz J. R. Tetrahedron. 2008;64:2011–2026.
-
- Frisone M. D. T. Pinna F. Strukul G. Organometallics. 1993;12:148–156.
Publication types
LinkOut - more resources
Full Text Sources

