Baeyer-Villiger oxidation: a promising tool for the synthesis of natural products: a review
- PMID: 39055269
- PMCID: PMC11270005
- DOI: 10.1039/d4ra03914a
Baeyer-Villiger oxidation: a promising tool for the synthesis of natural products: a review
Abstract
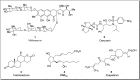
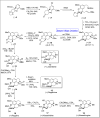
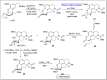
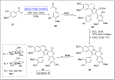
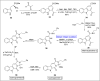
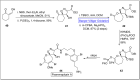
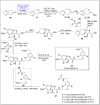
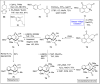
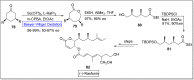
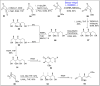
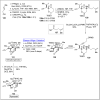
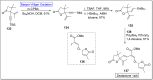
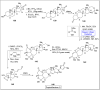
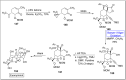
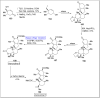
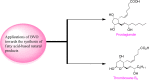
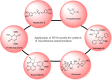
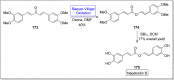
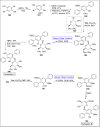
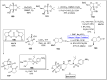
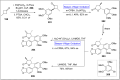
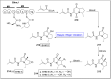
Baeyer-Villiger oxidation is a well-known reaction utilized for the synthesis of lactones and ester functionalities from ketones. Chiral lactones can be synthesized from chiral or racemic ketones by employing asymmetric Baeyer-Villiger oxidation. These lactones act as key intermediates in the synthesis of most of the biologically active natural products, their analogues, and derivatives. Various monooxygenases and oxidizing agents facilitate BV oxidation, providing a broad range of synthetic applications in organic chemistry. The variety of enzymatic and chemoselective Baeyer-Villiger oxidations and their substantial role in the synthesis of natural products i.e., alkaloids, polyketides, fatty acids, terpenoids, etc. (reported since 2018) have been summarized in this review article.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures










References
-
- Cavarzan A. Bianchini G. Sgarbossa P. Lefort L. Gladiali S. Scarso A. Strukul G. Chem.–Eur. J. 2009;15:7930–7939. - PubMed
-
- Leisch H. Morley K. Lau P. C. Chem. Rev. 2011;111:4165–4222. - PubMed
-
- Jiménez-Sanchidrián C. Ruiz J. R. Tetrahedron. 2008;64:2011–2026.
-
- Frisone M. D. T. Pinna F. Strukul G. Organometallics. 1993;12:148–156.
Publication types
LinkOut - more resources
Full Text Sources

