Effect of Pulsed Low-Level Lasers on Adult versus Neonatal Human Retinal Pigment Epithelial Cells: An in-vitro Study
- PMID: 39055499
- PMCID: PMC11267131
- DOI: 10.18502/jovr.v19i2.13278
Effect of Pulsed Low-Level Lasers on Adult versus Neonatal Human Retinal Pigment Epithelial Cells: An in-vitro Study
Abstract
Purpose: To investigate the short-term effects of low-level lasers (LLLs; also known as low-power laser therapy) on the structure, genetic, and phenotype of cultured human retinal pigment epithelial (hRPE) cells from both adult and neonatal sources.
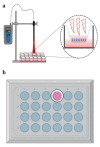
Methods: Cultivated adult and neonatal hRPE cells were irradiated with two types of LLL (630 nm and 780 nm), 1 min daily for five consecutive days.
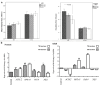
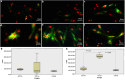
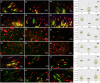
Results: An increase in doubling time was observed in 630 nm-irradiated adult hRPE cells (P = 0.032). The gene expression profile revealed increased expression of retinoid isomerohydrolase RPE65 (RPE65) (P 0.01 for 630 nm laser, P 0.001 for 780 nm laser) and nestin (NES) (P 0.01 for 630 nm laser) in neonatal hRPE cells, upregulation of RPE65 (P 0.001 for 780 nm laser) and paired box 6 (PAX6) (P 0.001 for 780 nm laser) genes in adult hRPE cells, and reduced expression of actin alpha 2 (ACTA2) in 780 nm-irradiated adult hRPE cells (P 0.001). Except the significant increase of -SMA in 780 nm-irradiated neonatal hRPE cells, no significant change was noted in the expressions of other investigated proteins.
Conclusion: Short-term irradiation of neonatal and adult hRPE cells with LLLs may induce multipotency at the transcriptional level. Irradiation of neonatal hRPE cells with LLLs can be associated with increased risk of myofibroblastic transformation; however, adult hRPE cells irradiated with the 780 nm laser have minimal risk of myofibroblastic differentiation. It seems that the 780 nm laser may be a promising option for future photobiomodulation in retinal degenerations in adults.
Keywords: Human Retinal Pigment Epithelial Cells; Low-level Laser; PAX6; Photobiomodulation; RPE65; Alpha-smooth Muscle Actin.
Copyright © 2024 Moshtaghion et al.
Conflict of interest statement
None.
Figures


References
-
- Morten la Cour TT. The retinal pigment epithelium. Adv Oral Biol. 2005;10:253–272.
-
- Jin ZB, Gao ML, Deng WL, Wu KC, Sugita S, Mandai M, et al. Stemming retinal regeneration with pluripotent stem cells. Stem Cell Res Ther. 2019;10:38–56. - PubMed
-
- Atmaca-Sonmez P, Li Y, Yamauchi Y, Schanie CL, Ildstad ST, Kaplan HJ, et al. Systemically transferred hematopoietic stem cells home to the subretinal space and express RPE-65 in a mouse model of retinal pigment epithelium damage. Exp Eye Res. 2006;83:1295–1302. - PubMed
-
- Liang CM, Tai MC, Chang YH, Chen YH, Chen CL, Lu DW, et al. Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy. Acta Ophthalmol. 2011;89:e505–14. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
