Case report: Pathological complete response to neoadjuvant brigatinib in stage III non-small cell lung cancer with ALK rearrangement
- PMID: 39055554
- PMCID: PMC11269150
- DOI: 10.3389/fonc.2024.1343238
Case report: Pathological complete response to neoadjuvant brigatinib in stage III non-small cell lung cancer with ALK rearrangement
Abstract
Purpose: The use of neoadjuvant anaplastic lymphoma kinase (ALK)-tyrosine kinase inhibitors (TKIs) has not been extensively explored. The current case report highlights the notable pathological complete response (pCR) achieved following neoadjuvant brigatinib therapy in a patient with stage IIIA ALK-positive non-small cell lung cancer (NSCLC).
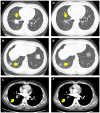
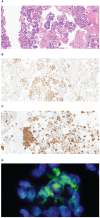
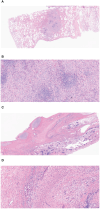
Case presentation: A 32-year-old male presented with incidental lung lesions, ultimately diagnosed as clinical stage T3N1M0, IIIA NSCLC with an ALK gene rearrangement. Following a multidisciplinary discussion, the patient opted for neoadjuvant brigatinib therapy, which significantly reduced the tumor size. Subsequently, surgery with curative intent was performed, revealing pCR with no residual tumor cells. The patient remained disease-free during a 13-month follow-up period.
Conclusion: This case report provides compelling evidence of pCR following brigatinib therapy in ALK-positive NSCLC, suggesting that surgery after neoadjuvant therapy with brigatinib may offer a safe and effective approach for patients with ALK-positive NSCLC.
Keywords: anaplastic lymphoma kinase-tyrosine kinase inhibitor; brigatinib; case report; lung cancer; neoadjuvant treatment.
Copyright © 2024 Seong, Kim, Kim, Cho, Kim and Eom.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. . Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. (2020) 31:1056–64. doi: 10.1016/j.annonc.2020.04.478 - DOI - PubMed
-
- Lv C, Fang W, Wu N, Jiao W, Xu S, Ma H, et al. . Osimertinib as neoadjuvant therapy in patients with EGFR-mutant resectable stage II-IIIB lung adenocarcinoma (NEOS): A multicenter, single-arm, open-label phase 2b trial. Lung Cancer. (2023) 178:151–6. doi: 10.1016/j.lungcan.2023.02.011 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

