Case report: Metastatic refractory undifferentiated small round-cell sarcoma successfully treated with surufatinib and camrelizumab
- PMID: 39055564
- PMCID: PMC11269156
- DOI: 10.3389/fonc.2024.1416241
Case report: Metastatic refractory undifferentiated small round-cell sarcoma successfully treated with surufatinib and camrelizumab
Abstract
Background: Undifferentiated small round-cell sarcomas (uSRCSs) are a subgroup of sarcomas that are difficult to diagnose. Some uSRCSs have specific gene re-arrangements, but others do not. Currently, there is no specific treatments for advanced uSRCSs, and its treatment is largely based on general experience with sarcomas, which includes chemotherapy, targeted therapy, and immunotherapy. In this article, we report a patient with uSRCS who responded to treatment with anti-VEGF inhibitor surufatinib and anti-PD-1 inhibitor camrelizumab after progression on first-line chemotherapy, second-line anlotinib combined with immunotherapy, and third-line chemotherapy.
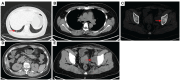
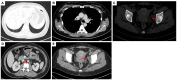
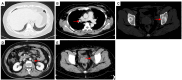
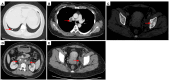
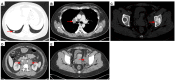
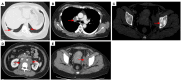
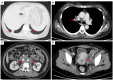
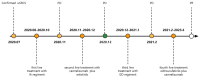
Case description: In July 2020, a 37-year-old female patient was diagnosed with advanced uSRCS. Results for the Ewing sarcoma RNA binding protein 1 and Wilms tumor suppressor (EWSR1/WT1) fusion gene were negative. The patient was also negative with BCOR (BCL6 co-repressor) and CIC (capicua transcriptional repressor) fusion gene. The next-generation sequencing results revealed point mutations on Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Beta (PIK3CB), Transcription Factor Binding To IGHM Enhancer 3 (TFE3), Mucin 16 (MUC16), and AXL (Axl, also called UFO, ARK, and Tyro7, is part of a family of receptor tyrosine kinases). The patient received 4 cycles of the Ifosfamide and epirubicin hydrochloride regimen, and her best objective response was stable disease. On November 3, 2020, a computed tomography (CT) scan revealed progressive disease (PD). Two cycles of camrelizumab (a programmed death-1 inhibitor) plus anlotinib (an anti- vascular endothelial growth factor drug) were administered, but PD was again observed. Thus, a regimen of gemcitabine plus docetaxel was adopted. Unfortunately, the disease progressed once again after two cycles of the treatment. On February 4, 2021, the patient began to receive targeted therapy with surufatinib combined with camrelizumab. A CT scan showed that the tumor achieved a partial response. As of April 2023, the patient had a progression-free survival time of 26 months.
Conclusions: Surufatinib in combination with camrelizumab could be effective in the treatment of advanced uSRCSs.
Keywords: case report; programmed death-1 inhibitor; surufatinib; target therapy; undifferentiated small round-cell sarcoma.
Copyright © 2024 Li, Huang, Chen, Ye, Du, Voutsadakis, Seetharam, Zhang and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- WHO Classification of Tumours Editorial Board. Press I: WHO classification of tumours editorial board. In: Soft Tissue and Bone Tumours, 5th edn, vol. 3. SWISS: World Health Organization.
-
- Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, et al. . Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. (2017) 18:1397–410. doi: 10.1016/S1470-2045(17)30622-8 - DOI - PMC - PubMed
-
- Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. . Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. (2016) 17:1732–42. doi: 10.1016/S1470-2045(16)30507-1 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

