Parkinson-like neurotoxicity in female patients treated with idecabtagene-vicleucel
- PMID: 39055647
- PMCID: PMC11270580
- DOI: 10.1002/hem3.131
Parkinson-like neurotoxicity in female patients treated with idecabtagene-vicleucel
Conflict of interest statement
Roch Houot received honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, and Roche; and consultancy at Kite/Gilead, 63 Novartis, Bristol‐Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi. Olivier Decaux received honoraria from Janssen, Celgene/BMS, Amgen, Takeda, GSK, Sanofi, Abbvie, Roche, The Binding Site, Sebia, Menarini‐Stemline, and Pfizer. The remaining authors declare no competing financial interests.
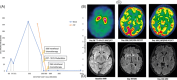
Figures
References
LinkOut - more resources
Full Text Sources
