Anti-inflammatory effect of semaglutide: updated systematic review and meta-analysis
- PMID: 39055657
- PMCID: PMC11270812
- DOI: 10.3389/fcvm.2024.1379189
Anti-inflammatory effect of semaglutide: updated systematic review and meta-analysis
Abstract
Background: The anti-inflammatory effect could be one of the mechanisms by which semaglutide reduces cardiovascular risk in patients with type 2 diabetes mellitus (T2DM) and/or obesity. Determining the anti-inflammatory effect of semaglutide was the objective of this systematic review and meta-analysis.
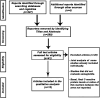
Methods: This meta-analysis was performed according to the PRISMA guidelines. A literature search was performed to detect randomised clinical trials that have quantified the effect of semaglutide on C-reactive protein (CRP) levels compared to placebo or a control group (other glucose-lowering drugs). The primary outcome was CRP index (final CRP/basal CRP). A random-effects model was used.
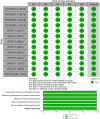
Results: Thirteen randomised clinical trials were considered eligible (n = 26,131). Overall, semaglutide therapy was associated with lower CRP index values compared to the placebo group (SMD -0.56; 95% CI -0.69 to -0.43, I 2 92%) or the control group (SMD -0.45; 95% CI -0.68 to -0.23, I 2 82%).Such an association was similarly observed when different treatment regimens (subcutaneous vs. oral) or different populations (patients with or without T2DM) were analysed. The sensitivity analysis showed that the results were robust.
Conclusion: The present meta-analysis demonstrated that the use of semaglutide was associated with a reduction in inflammation irrespective of the population evaluated or the treatment regimen used. These findings would explain one of the mechanisms by which semaglutide reduces cardiovascular events.
Systematic review registration: PROSPERO [CRD42024500551].
Keywords: C-reactive protein; glucagon-like peptide-1 receptor agonists; inflammation; meta-analysis; semaglutide.
© 2024 Masson, Lobo, Nogueira, Rodriguez-Granillo, Barbagelata and Siniawski.
Conflict of interest statement
WM, ML and DS have served as a speaker from Novo Nordisk. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Patti AM, Nikolic D, Magan-Fernandez A, Giglio RV, Castellino G, Chianetta R, et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: an 8-month prospective study. Diabetes Res Clin Pract. (2019) 149:163–9. 10.1016/j.diabres.2019.02.006 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

