Novel biomarkers for prediction of atonic postpartum hemorrhage among 'low-risk' women in labor
- PMID: 39055706
- PMCID: PMC11269088
- DOI: 10.3389/fimmu.2024.1416990
Novel biomarkers for prediction of atonic postpartum hemorrhage among 'low-risk' women in labor
Abstract
Background: Postpartum hemorrhage (PPH) is the primary cause of maternal mortality globally, with uterine atony being the predominant contributing factor. However, accurate prediction of PPH in the general population remains challenging due to a lack of reliable biomarkers.
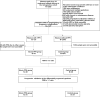
Methods: Using retrospective cohort data, we quantified 48 cytokines in plasma samples from 40 women diagnosed with PPH caused by uterine atony. We also analyzed previously reported hemogram and coagulation parameters related to inflammatory response. The least absolute shrinkage and selection operator (LASSO) and logistic regression were applied to develop predictive models. Established models were further evaluated and temporally validated in a prospective cohort.
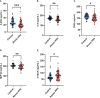
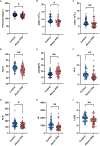
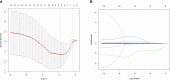
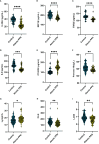
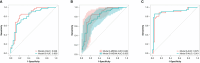
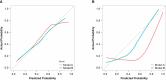
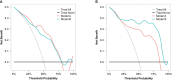
Results: Fourteen factors showed significant differences between the two groups, among which IL2Rα, IL9, MIP1β, TNFβ, CTACK, prenatal Hb, Lymph%, PLR, and LnSII were selected by LASSO to construct predictive model A. Further, by logistic regression, model B was constructed using prenatal Hb, PLR, IL2Rα, and IL9. The area under the curve (AUC) values of model A in the training set, internal validation set, and temporal validation set were 0.846 (0.757-0.934), 0.846 (0.749-0.930), and 0.875 (0.789-0.961), respectively. And the corresponding AUC values for model B were 0.805 (0.709-0.901), 0.805 (0.701-0.894), and 0.901 (0.824-0.979). Decision curve analysis results showed that both nomograms had a high net benefit for predicting atonic PPH.
Conclusion: We identified novel biomarkers and developed predictive models for atonic PPH in women undergoing "low-risk" vaginal delivery, providing immunological insights for further exploration of the mechanism underlying atonic PPH.
Keywords: atonic postpartum hemorrhage; biomarker; coagulation; cytokine; hemogram; prediction.
Copyright © 2024 Zhang, Jia, Song, Fan, Lv, Geng, Zhao, Cui and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

