Development of PROTACS degrading KRAS and SOS1
- PMID: 39055890
- PMCID: PMC11267056
- DOI: 10.32604/or.2024.051653
Development of PROTACS degrading KRAS and SOS1
Abstract
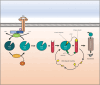
The Kirsten rat sarcoma virus-son of sevenless 1 (KRAS-SOS1) axis drives tumor growth preferentially in pancreatic, colon, and lung cancer. Now, KRAS G12C mutated tumors can be successfully treated with inhibitors that covalently block the cysteine of the switch II binding pocket of KRAS. However, the range of other KRAS mutations is not amenable to treatment and the G12C-directed agents Sotorasib and Adragrasib show a response rate of only approximately 40%, lasting for a mean period of 8 months. One approach to increase the efficacy of inhibitors is their inclusion into proteolysis-targeting chimeras (PROTACs), which degrade the proteins of interest and exhibit much higher antitumor activity through multiple cycles of activity. Accordingly, PROTACs have been developed based on KRAS- or SOS1-directed inhibitors coupled to either von Hippel-Lindau (VHL) or Cereblon (CRBN) ligands that invoke the proteasomal degradation. Several of these PROTACs show increased activity in vitro and in vivo compared to their cognate inhibitors but their toxicity in normal tissues is not clear. The CRBN PROTACs containing thalidomide derivatives cannot be tested in experimental animals. Resistance to such PROTACS arises through downregulation or inactivation of CRBN or factors of the functional VHL E3 ubiquitin ligase. Although highly active KRAS and SOS1 PROTACs have been formulated their clinical application remains difficult.
Keywords: Cereblon; Kirsten rat sarcoma virus (KRAS); Proteolysis-targeting chimeras (PROTACs); Son of sevenless 1 (SOS1); Von Hippel-Lindau.
© 2024 Hamilton, Eggerstorfer and Stickler.
Conflict of interest statement
The authors declare that they have no conflicts of interest to report regarding the present study.
Figures
Similar articles
-
An overview of PROTACs targeting KRAS and SOS1 as antitumor agents.Bioorg Med Chem Lett. 2025 Sep 1;125-126:130283. doi: 10.1016/j.bmcl.2025.130283. Epub 2025 May 15. Bioorg Med Chem Lett. 2025. PMID: 40381703 Review.
-
Targeting of SOS1: from SOS1 Activators to Proteolysis Targeting Chimeras.Curr Pharm Des. 2023;29(22):1741-1746. doi: 10.2174/1381612829666230418114520. Curr Pharm Des. 2023. PMID: 37073657
-
Cereblon versus VHL: Hijacking E3 ligases against each other using PROTACs.Bioorg Med Chem. 2019 Jun 15;27(12):2466-2479. doi: 10.1016/j.bmc.2019.02.048. Epub 2019 Feb 22. Bioorg Med Chem. 2019. PMID: 30826187 Free PMC article.
-
CRBN-PROTACs in Cancer Therapy: From Mechanistic Insights to Clinical Applications.Chem Biol Drug Des. 2024 Nov;104(5):e70009. doi: 10.1111/cbdd.70009. Chem Biol Drug Des. 2024. PMID: 39496477 Review.
-
The SOS1 Inhibitor MRTX0902 Blocks KRAS Activation and Demonstrates Antitumor Activity in Cancers Dependent on KRAS Nucleotide Loading.Mol Cancer Ther. 2024 Oct 1;23(10):1418-1430. doi: 10.1158/1535-7163.MCT-23-0870. Mol Cancer Ther. 2024. PMID: 38904222 Free PMC article.
Cited by
-
KRAS mutated NSCLC: past, present, and future directions in a rapidly evolving landscape.Oncologist. 2025 Jun 4;30(6):oyaf153. doi: 10.1093/oncolo/oyaf153. Oncologist. 2025. PMID: 40586765 Free PMC article. Review.
-
Protein lipidation in the tumor microenvironment: enzymology, signaling pathways, and therapeutics.Mol Cancer. 2025 May 7;24(1):138. doi: 10.1186/s12943-025-02309-7. Mol Cancer. 2025. PMID: 40335986 Free PMC article. Review.
References
-
- Ou, S. I., Jänne, P. A., Leal, T. A., Rybkin, I. I., Sabari, J. K.et al. (2022). First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRASG12C solid tumors (KRYSTAL-1). Journal of Clinical Oncology , 40(23), 2530–2538. 10.1200/JCO.21.02752; - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
