The immunosuppressive tuberculosis-associated microenvironment inhibits viral replication and promotes HIV-1 latency in CD4+ T cells
- PMID: 39055929
- PMCID: PMC11269811
- DOI: 10.1016/j.isci.2024.110324
The immunosuppressive tuberculosis-associated microenvironment inhibits viral replication and promotes HIV-1 latency in CD4+ T cells
Abstract
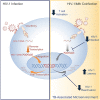
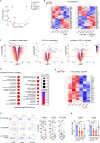
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), is the most common coinfection among people living with HIV-1. This coinfection is associated with accelerated HIV-1 disease progression and reduced survival. However, the impact of the HIV-1/TB coinfection on HIV-1 replication and latency in CD4+ T cells remains poorly studied. Using the acellular fraction of tuberculous pleural effusion (TB-PE), we investigated whether viral replication and HIV-1 latency in CD4+ T cells are affected by a TB-associated microenvironment. Our results revealed that TB-PE impaired T cell receptor-dependent cell activation and decreased HIV-1 replication in CD4+ T cells. Moreover, this immunosuppressive TB microenvironment promoted viral latency and inhibited HIV-1 reactivation. This study indicates that the TB-induced immune response may contribute to the persistence of the viral reservoir by silencing HIV-1 expression, allowing the virus to persist undetected by the immune system, and increasing the size of the latent HIV-1 reservoir.
Keywords: Immunology; Virology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- WHO HIV and AIDS Fact sheet. 2022. https://www.who.int/news-room/fact-sheets/detail/hiv-aids
-
- Chun T.W., Stuyver L., Mizell S.B., Ehler L.A., Mican J.A., Baseler M., Lloyd A.L., Nowak M.A., Fauci A.S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. - DOI - PMC - PubMed
-
- Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E., Quinn T.C., Chadwick K., Margolick J., Brookmeyer R., et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

