Clinicopathologic Characterization of 2 Individuals With TBK1 Variants-1 Novel Splice Variant, 2 Proteinopathies: A Case Series
- PMID: 39055961
- PMCID: PMC11270891
- DOI: 10.1212/NXG.0000000000200173
Clinicopathologic Characterization of 2 Individuals With TBK1 Variants-1 Novel Splice Variant, 2 Proteinopathies: A Case Series
Abstract
Objectives: Here, we report detailed clinicopathologic evaluation of 2 individuals with pathogenic variants in TBK1, including one novel likely pathogenic splice variant. We describe the striking diversity of clinical phenotypes among family members and also the brain and spinal cord neuropathology associated with these 2 distinct TBK1 variants.
Methods: Two individuals with pathogenic variants in TBK1 and their families were clinically characterized, and the probands subsequently underwent extensive postmortem neuropathologic examination of their brains and spinal cords.
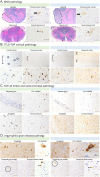
Results: Multiple affected individuals within a single family were found to carry a previously unreported c.358+3A>G variant, predicted to alter splicing. Detailed histopathologic evaluation of our 2 TBK1 variant carriers demonstrated distinct TDP-43 pathologic subtypes, but shared argyrophilic grain disease (AGD) tau pathology.
Discussion: Although all pathogenic TBK1 variants are associated with TDP-43 pathology, the clinical and histologic features can be highly variable. Within one family, we describe distinct neurologic presentations which we propose are all caused by a novel c.358+3A>G variant. AGD is typically associated with older age, but it has been described as a copathologic finding in other TBK1 variant carriers and may be a common feature in FTLD-TDP due to TBK1.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
K. Domoto-Reilly reports research funding from NIH U19AG063911; B.J. Distad reports no disclosures relevant to the manuscript; D.E. Miller reports participation on a scientific advisory board at Oxford Nanopore Technologies (ONT), engagement in a research agreement with ONT, and has received travel compensation from ONT; Y.-H. Lin reports no disclosures relevant to the manuscript; D. Ivanick reports no disclosures relevant to the manuscript; A.S. Warren reports no disclosures relevant to the manuscript; S. Jayadev reports no disclosures relevant to the manuscript; C. Latimer reports no disclosures relevant to the manuscript. Go to Neurology.org/NG for full disclosures.
Figures

References
-
- Hirsch-Reinshagen V, Alfaify OA, Hsiung G-YR, et al. . Clinicopathologic correlations in a family with a TBK1 mutation presenting as primary progressive aphasia and primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7-8):568-575. doi:10.1080/21678421.2019.1632347 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
