Patterns of brain volume and metabolism predict clinical features in the progressive supranuclear palsy spectrum
- PMID: 39056025
- PMCID: PMC11272075
- DOI: 10.1093/braincomms/fcae233
Patterns of brain volume and metabolism predict clinical features in the progressive supranuclear palsy spectrum
Abstract
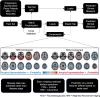
Progressive supranuclear palsy (PSP) is a neurodegenerative tauopathy that presents with highly heterogenous clinical syndromes. We perform cross-sectional data-driven discovery of independent patterns of brain atrophy and hypometabolism across the entire PSP spectrum. We then use these patterns to predict specific clinical features and to assess their relationship to phenotypic heterogeneity. We included 111 patients with PSP (60 with Richardson syndrome and 51 with cortical and subcortical variant subtypes). Ninety-one were used as the training set and 20 as a test set. The presence and severity of granular clinical variables such as postural instability, parkinsonism, apraxia and supranuclear gaze palsy were noted. Domains of akinesia, ocular motor impairment, postural instability and cognitive dysfunction as defined by the Movement Disorders Society criteria for PSP were also recorded. Non-negative matrix factorization was used on cross-sectional MRI and fluorodeoxyglucose-positron emission tomography (FDG-PET) scans. Independent models for each as well as a combined model for MRI and FDG-PET were developed and used to predict the granular clinical variables. Both MRI and FDG-PET were better at predicting presence of a symptom than severity, suggesting identification of disease state may be more robust than disease stage. FDG-PET predicted predominantly cortical abnormalities better than MRI such as ideomotor apraxia, apraxia of speech and frontal dysexecutive syndrome. MRI demonstrated prediction of cortical and more so sub-cortical abnormalities, such as parkinsonism. Distinct neuroanatomical foci were predictive in MRI- and FDG-PET-based models. For example, vertical gaze palsy was predicted by midbrain atrophy on MRI, but frontal eye field hypometabolism on FDG-PET. Findings also differed by scale or instrument used. For example, prediction of ocular motor abnormalities using the PSP Saccadic Impairment Scale was stronger than with the Movement Disorders Society Diagnostic criteria for PSP oculomotor impairment designation. Combination of MRI and FDG-PET demonstrated enhanced detection of parkinsonism and frontal syndrome presence and apraxia, cognitive impairment and bradykinesia severity. Both MRI and FDG-PET patterns were able to predict some measures in the test set; however, prediction of global cognition measured by Montreal Cognitive Assessment was the strongest. MRI predictions generalized more robustly to the test set. PSP leads to neurodegeneration in motor, cognitive and ocular motor networks at cortical and subcortical foci, leading to diverse yet overlapping clinical syndromes. To advance understanding of phenotypic heterogeneity in PSP, it is essential to consider data-driven approaches to clinical neuroimaging analyses.
Keywords: diagnosis; machine learning; neurodegeneration; neuroimaging; tauopathy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Litvan I, Agid Y, Jankovic J, et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology. 1996;46(4):922–930. - PubMed
-
- Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44(11):2015–2019. - PubMed
-
- Respondek G, Höglinger GU. The phenotypic spectrum of progressive supranuclear palsy. Parkinsonism Relat Disord. 2016;22(Suppl 1):S34–S36. - PubMed
-
- Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333–359. - PubMed
-
- Donker Kaat L, Boon AJW, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC. Frontal presentation in progressive supranuclear palsy. Neurology. 2007;69(8):723–729. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
