Crucial role for sensory nerves and Na/H exchanger inhibition in dapagliflozin- and empagliflozin-induced arterial relaxation
- PMID: 39056245
- PMCID: PMC11587556
- DOI: 10.1093/cvr/cvae156
Crucial role for sensory nerves and Na/H exchanger inhibition in dapagliflozin- and empagliflozin-induced arterial relaxation
Abstract
Aims: Sodium/glucose transporter 2 (SGLT2 or SLC5A2) inhibitors lower blood glucose and are also approved treatments for heart failure independent of raised glucose. Various studies have showed that SGLT2 inhibitors relax arteries, but the underlying mechanisms are poorly understood and responses variable across arterial beds. We speculated that SGLT2 inhibitor-mediated arterial relaxation is dependent upon calcitonin gene-related peptide (CGRP) released from sensory nerves independent of glucose transport.
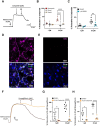
Methods and results: The functional effects of SGLT1 and 2 inhibitors (mizagliflozin, dapagliflozin, and empagliflozin) and the sodium/hydrogen exchanger 1 (NHE1) blocker cariporide were determined on pre-contracted resistance arteries (mesenteric and cardiac septal arteries) as well as main renal conduit arteries from male Wistar rats using wire myography. SGLT2, CGRP, TRPV1, and NHE1 expression was determined by western blot and immunohistochemistry. Kv7.4/5/KCNE4 and TRPV1 currents were measured in the presence and absence of dapagliflozin and empagliflozin. All SGLT inhibitors (1-100 µM) and cariporide (30 µM) relaxed mesenteric arteries but had negligible effect on renal or septal arteries. Immunohistochemistry with TRPV1 and CGRP antibodies revealed a dense innervation of sensory nerves in mesenteric arteries that were absent in renal and septal arteries. Consistent with a greater sensory nerve component, the TRPV1 agonist capsaicin relaxed mesenteric arteries more effectively than renal or septal arteries. In mesenteric arteries, relaxations to dapagliflozin, empagliflozin, and cariporide were attenuated by the CGRP receptor antagonist BIBN-4096, depletion of sensory nerves with capsaicin, and blockade of TRPV1 or Kv7 channels. Neither dapagliflozin nor empagliflozin activated heterologously expressed TRPV1 channels or Kv7 channels directly. Sensory nerves also expressed NHE1 but not SGLT2 and cariporide pre-application as well as knockdown of NHE1 by translation stop morpholinos prevented the relaxant response to SGLT2 inhibitors.
Conclusion: SGLT2 inhibitors relax mesenteric arteries by promoting the release of CGRP from sensory nerves in a NHE1-dependent manner.
Keywords: Calcitonin-gene related peptide; Sensory nerves; Sodium/glucose transporter 2; Sodium/hydrogen exchanger; Vasodilatation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures







References
-
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 2012;14:83–90. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, Boer RA, de Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. NEJM 2019;381:1995–2008. - PubMed
-
- Nassif ME, Windsor S, Tang F, Khariton Y, Husain M, Inzucchi S, McGuire D, Pitt B, Scirica B, Austin B, Drazner M, Fong M, Givertz M, Gordon R, Jermyn R, Katz S, Lamba S, Lanfear D, LaRue S, Lindenfeld JA, Malone M, Margulies K, Mentz R, Kannan Mutharasan R, Pursley M, Umpierrez G, Kosiborod M, Malik A, Wenger N, Ogunniyi M, Vellanki P, Murphy B, Newman J, Hartupee J, Gupta C, Goldsmith M, Baweja P, Montero M, Gottlieb S, Costanzo MR, Hoang T, Warnock A, Allen L, Tang W, Chen H, Cox J. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction. Circulation 2019;140:1463–1476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

