Dosing and Safety Profile of Aficamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy: Results From SEQUOIA-HCM
- PMID: 39056349
- PMCID: PMC11964075
- DOI: 10.1161/JAHA.124.035993
Dosing and Safety Profile of Aficamten in Symptomatic Obstructive Hypertrophic Cardiomyopathy: Results From SEQUOIA-HCM
Abstract
Background: Aficamten, a novel cardiac myosin inhibitor, reversibly reduces cardiac hypercontractility in obstructive hypertrophic cardiomyopathy. We present a prespecified analysis of the pharmacokinetics, pharmacodynamics, and safety of aficamten in SEQUOIA-HCM (Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of Aficamten in HCM).
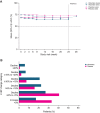
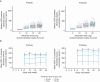
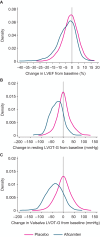
Methods and results: A total of 282 patients with obstructive hypertrophic cardiomyopathy were randomized 1:1 to daily aficamten (5-20 mg) or placebo between February 1, 2022, and May 15, 2023. Aficamten dosing targeted the lowest effective dose for achieving site-interpreted Valsalva left ventricular outflow tract gradient <30 mm Hg with left ventricular ejection fraction (LVEF) ≥50%. End points were evaluated during titration (day 1 to week 8), maintenance (weeks 8-24), and washout (weeks 24-28), and included major adverse cardiac events, new-onset atrial fibrillation, implantable cardioverter-defibrillator discharges, LVEF <50%, and treatment-emergent adverse events. At week 8, 3.6%, 12.9%, 35%, and 48.6% of patients achieved 5-, 10-, 15-, and 20-mg doses, respectively. Baseline characteristics were similar across groups. Aficamten concentration increased by dose and remained stable during maintenance. During the treatment period, LVEF decreased by -0.9% (95% CI, -1.3 to -0.6) per 100 ng/mL aficamten exposure. Seven (4.9%) patients taking aficamten underwent per-protocol dose reduction for site-interpreted LVEF <50%. There were no treatment interruptions or heart failure worsening for LVEF <50%. No major adverse cardiovascular events were associated with aficamten, and treatment-emergent adverse events were similar between treatment groups, including atrial fibrillation.
Conclusions: A site-based dosing algorithm targeting the lowest effective aficamten dose reduced left ventricular outflow tract gradient with a favorable safety profile throughout SEQUOIA-HCM.
Registration: URL: https://www.clinicaltrials.gov; Unique Identifier: NCT05186818.
Keywords: aficamten; cardiac myosin inhibitor; hypertrophic cardiomyopathy.
Figures

References
-
- Desai MY, Owens A, Wolski K, Geske JB, Saberi S, Wang A, Sherrid M, Cremer PC, Lakdawala NK, Tower‐Rader A, et al. Mavacamten in patients with hypertrophic cardiomyopathy referred for septal reduction: week 56 results from the VALOR‐HCM randomized clinical trial. JAMA Cardiol. 2023;8:968–977. doi: 10.1001/jamacardio.2023.3342 - DOI - PMC - PubMed
-
- Olivotto I, Oreziak A, Barriales‐Villa R, Abraham TP, Masri A, Garcia‐Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER‐HCM): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X - DOI - PubMed
-
- Rader F, Oreziak A, Choudhury L, Saberi S, Fermin D, Wheeler MT, Abraham TP, Garcia‐Pavia P, Zwas DR, Masri A, et al. Mavacamten treatment for symptomatic obstructive hypertrophic cardiomyopathy: interim results from the MAVA‐LTE study, EXPLORER‐LTE cohort. JACC Heart Fail. 2024;12:164–177. doi: 10.1016/j.jchf.2023.09.028 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

