Boron-Nitrogen-Containing Benzene Valence Isomers
- PMID: 39056374
- PMCID: PMC12092074
- DOI: 10.1002/chem.202402544
Boron-Nitrogen-Containing Benzene Valence Isomers
Abstract
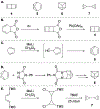
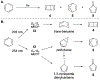
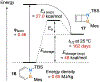
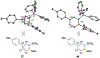
Benzene is one of the most ubiquitous structural motifs in chemistry. The valence isomers of benzene have also attracted synthetic chemists' attention due to their unique structures, bonding, and reactivity. We have been investigating boron-nitrogen-containing benzene valence isomers via photoisomerization of 1,2-azaborines. In this contribution, we summarize recent developments of these highly strained BN-heterocyclic compounds including their synthesis, characterization, proposed mechanism of formation, and their potential applications.
Keywords: Azaborines; BN-heterocycle; Benzvalene; Dewar benzene; Photoisomerization.
© 2024 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interests
The authors declare no conflict of interest.
Figures
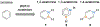
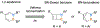
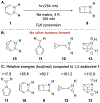



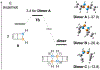
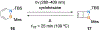
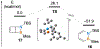
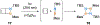
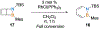
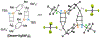

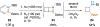




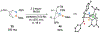
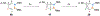
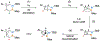
References
-
- Langmuir I, J. Am. Chem. Soc 1919, 41, 1543–1559.
-
- Bosdet MJD, Piers WE, Can. J. Chem 2009, 87, 8–29;
- Campbell PG, Marwitz AJV, Liu S-Y, Angew. Chem. Int. Ed 2012, 51, 6074–6092; - PMC - PubMed
- Bélanger-Chabot G, Braunschweig H, Roy DK, Eur. J. Inorg. Chem 2017, 4353–4368;
- Giustra ZX, Liu S-Y, J. Am. Chem. Soc 2018, 140, 1184–1194; - PMC - PubMed
- McConnell CR, Liu S-Y, Chem. Soc. Rev 2019, 48, 3436–3453. - PMC - PubMed
-
- Dewar MJS, Marr, P. A. J. Am. Chem. Soc 1962, 84, 3782–3782;
- White DG, J. Am. Chem. Soc 1963, 85, 3634–3636;
- Marwitz AJV, Matus MH, Zakharov N, Dixon DA, Liu S-Y, Angew. Chem. Int. Ed 2009, 48, 973–977. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

