Network signatures define consciousness state during focal seizures
- PMID: 39056406
- PMCID: PMC11534508
- DOI: 10.1111/epi.18074
Network signatures define consciousness state during focal seizures
Abstract
Objective: Epilepsy is a common neurological disorder affecting 1% of the global population. Loss of consciousness in focal impaired awareness seizures (FIASs) and focal-to-bilateral tonic-clonic seizures (FBTCSs) can be devastating, but the mechanisms are not well understood. Although ictal activity and interictal connectivity changes have been noted, the network states of focal aware seizures (FASs), FIASs, and FBTCSs have not been thoroughly evaluated with network measures ictally.
Methods: We obtained electrographic data from 74 patients with stereoelectroencephalography (SEEG). Sliding window band power, functional connectivity, and segregation were computed on preictal, ictal, and postictal data. Five-minute epochs of wake, rapid eye movement sleep, and deep sleep were also extracted. Connectivity of subcortical arousal structures was analyzed in a cohort of patients with both SEEG and functional magnetic resonance imaging (fMRI). Given that custom neuromodulation of seizures is predicated on detection of seizure type, a convolutional neural network was used to classify seizure types.
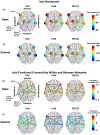
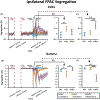
Results: We found that in the frontoparietal association cortex, an area associated with consciousness, both consciousness-impairing seizures (FIASs and FBTCSs) and deep sleep had increases in slow wave delta (1-4 Hz) band power. However, when network measures were employed, we found that only FIASs and deep sleep exhibited an increase in delta segregation and a decrease in gamma segregation. Furthermore, we found that only patients with FIASs had reduced subcortical-to-neocortical functional connectivity with fMRI versus controls. Finally, our deep learning network demonstrated an area under the curve of .75 for detecting consciousness-impairing seizures.
Significance: This study provides novel insights into ictal network measures in FASs, FIASs, and FBTCSs. Importantly, although both FIASs and FBTCSs result in loss of consciousness, our results suggest that ictal network changes in FIASs uniquely resemble those that occur during deep sleep. Our results may inform novel neuromodulation strategies for preservation of consciousness in epilepsy.
Keywords: consciousness‐impairing seizures; focal epilepsy; functional connectivity; network analysis of epilepsy; stereoelectroencephalography.
© 2024 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures



References
-
- Fisher RS. The new classification of seizures by the international league against epilepsy 2017. Curr Neurol Neurosci Rep. 2017;17:1–6. - PubMed
-
- Laureys S, Gosseries O, Tononi G. The neurology of consciousness: cognitive neuroscience and neuropathology. Cambridge, Massachusettes: Academic Press; 2015.
MeSH terms
Grants and funding
- T32GM007347/GM/NIGMS NIH HHS/United States
- R01NS112252/NS/NINDS NIH HHS/United States
- R01NS134625/NS/NINDS NIH HHS/United States
- F31NS120401/NS/NINDS NIH HHS/United States
- T32 EB021937/EB/NIBIB NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- F31NS131056/NS/NINDS NIH HHS/United States
- R01 NS108445/NS/NINDS NIH HHS/United States
- T32EB021937/EB/NIBIB NIH HHS/United States
- R01NS108445/NS/NINDS NIH HHS/United States
- F31 NS120401/NS/NINDS NIH HHS/United States
- R01 NS134625/NS/NINDS NIH HHS/United States
- R01 NS112252/NS/NINDS NIH HHS/United States
- T32 GM152284/GM/NIGMS NIH HHS/United States
- P50 HD103537/HD/NICHD NIH HHS/United States
- F31 NS131056/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

