Correlative Raman Imaging: Development and Cancer Applications
- PMID: 39056600
- PMCID: PMC11274409
- DOI: 10.3390/bios14070324
Correlative Raman Imaging: Development and Cancer Applications
Abstract
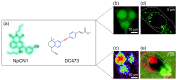
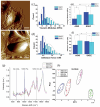
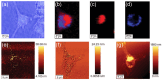
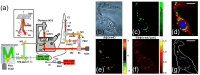
Despite extensive research efforts, cancer continues to stand as one of the leading causes of death on a global scale. To gain profound insights into the intricate mechanisms underlying cancer onset and progression, it is imperative to possess methodologies that allow the study of cancer cells at the single-cell level, focusing on critical parameters such as cell morphology, metabolism, and molecular characteristics. These insights are essential for effectively discerning between healthy and cancerous cells and comprehending tumoral progression. Recent advancements in microscopy techniques have significantly advanced the study of cancer cells, with Raman microspectroscopy (RM) emerging as a particularly powerful tool. Indeed, RM can provide both biochemical and spatial details at the single-cell level without the need for labels or causing disruptions to cell integrity. Moreover, RM can be correlated with other microscopy techniques, creating a synergy that offers a spectrum of complementary insights into cancer cell morphology and biology. This review aims to explore the correlation between RM and other microscopy techniques such as confocal fluoresce microscopy (CFM), atomic force microscopy (AFM), digital holography microscopy (DHM), and mass spectrometry imaging (MSI). Each of these techniques has their own strengths, providing different perspectives and parameters about cancer cell features. The correlation between information from these various analysis methods is a valuable tool for physicians and researchers, aiding in the comprehension of cancer cell morphology and biology, unraveling mechanisms underlying cancer progression, and facilitating the development of early diagnosis and/or monitoring cancer progression.
Keywords: Raman imaging; Raman spectroscopy; atomic force microscopy; cancer; correlative imaging; digital holography microscopy; fluorescence microscopy; mass spectroscopy imaging; quantitative phase imaging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

