Graft-Specific Regulatory T Cells for Long-Lasting, Local Tolerance Induction
- PMID: 39056797
- PMCID: PMC11274814
- DOI: 10.3390/cells13141216
Graft-Specific Regulatory T Cells for Long-Lasting, Local Tolerance Induction
Abstract
Background: Solid organ transplantation is hindered by immune-mediated chronic graft dysfunction and the side effects of immunosuppressive therapy. Regulatory T cells (Tregs) are crucial for modulating immune responses post-transplantation; however, the transfer of polyspecific Tregs alone is insufficient to induce allotolerance in rodent models.
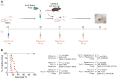
Methods: To enhance the efficacy of adoptive Treg therapy, we investigated different immune interventions in the recipients. By utilizing an immunogenic skin transplant model and existing transplantation medicine reagents, we facilitated the clinical translation of our findings. Specifically, antigen-specific Tregs were used.
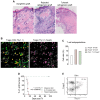
Results: Our study demonstrated that combining the available induction therapies with drug-induced T-cell proliferation due to lymphopenia effectively increased the Treg/T effector ratios. This results in significant Treg accumulation within the graft, leading to long-term tolerance after the transfer of antigen-specific Tregs. Importantly, all the animals achieved operational tolerance, which boosted the presence of adoptively transferred Tregs within the graft.
Conclusions: This protocol offers a means to establish tolerance by utilizing antigen-specific Tregs. These results have promising implications for future trials involving adoptive Treg therapy in organ transplantation.
Keywords: alloantigen-specific; immune tolerance; regulatory T cells; solid organ transplantation.
Conflict of interest statement
All authors declare that there are no conflicts of interest due to a desire for financial gain, prominence, professional advancement, or a successful outcome.
Figures

References
-
- Roemhild A., Otto N.M., Moll G., Abou-El-Enein M., Kaiser D., Bold G., Schachtner T., Choi M., Oellinger R., Landwehr-Kenzel S., et al. Regulatory T cells for minimising immune suppression in kidney transplantation: Phase I/IIa clinical trial. BMJ. 2020;371:m3734. doi: 10.1136/bmj.m3734. - DOI - PMC - PubMed
-
- Sato Y., Passerini L., Piening B.D., Uyeda M.J., Goodwin M., Gregori S., Snyder M.P., Bertaina A., Roncarolo M.G., Bacchetta R. Human-engineered Treg-like cells suppress FOXP3-deficient T cells but preserve adaptive immune responses in vivo. Clin. Transl. Immunol. 2020;9:e1214. doi: 10.1002/cti2.1214. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

