Advanced Kidney Models In Vitro Using the Established Cell Line Renal Proximal Tubular Epithelial/Telomerase Reverse Transcriptase1 for Nephrotoxicity Assays
- PMID: 39056887
- PMCID: PMC11275192
- DOI: 10.3390/biomimetics9070446
Advanced Kidney Models In Vitro Using the Established Cell Line Renal Proximal Tubular Epithelial/Telomerase Reverse Transcriptase1 for Nephrotoxicity Assays
Abstract
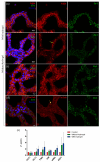
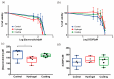
Nephrotoxicity stands as one of the most limiting effects in the development and validation of new drugs. The kidney, among the organs evaluated in toxicity assessments, has a higher susceptibility, with nephrotoxic potential frequently evading detection until late in clinical trials. Traditional cell culture, which has been widely used for decades, does not recapitulate the structure and complexity of the native tissue, which can affect cell function, and the response to cytotoxins does not resemble what occurs in the kidney. In the current study, we aimed to address these challenges by creating in vitro kidney models that faithfully biomimic the dynamics of the renal proximal tubule, using the well-established RPTEC/TERT1 cell line. For doing so, two models were developed, one recreating tubule-like structures (2.5D model) and the other using microfluidic technology (kidney-on-a-chip). The 2.5D model allowed tubular structures to be generated in the absence of hydrogels, and the kidney-on-a-chip model allowed shear stress to be applied to the cell culture, which is a physiological stimulus in the renal tissue. After characterization of both models, different nephrotoxic compounds such as cisplatin, tacrolimus, and daunorubicin were used to study cell responses after treatment. The developed models in our study could be a valuable tool for pre-clinical nephrotoxic testing of drugs and new compounds.
Keywords: 3D structures; hydrogel; in vitro models; kidney; kidney-on-a-chip; nephrotoxicity.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Functional transepithelial transport measurements to detect nephrotoxicity in vitro using the RPTEC/TERT1 cell line.Arch Toxicol. 2019 Jul;93(7):1965-1978. doi: 10.1007/s00204-019-02469-8. Epub 2019 May 10. Arch Toxicol. 2019. PMID: 31076804
-
RPTEC/TERT1 cells form highly differentiated tubules when cultured in a 3D matrix.ALTEX. 2018;35(2):223-234. doi: 10.14573/altex.1710181. Epub 2017 Dec 2. ALTEX. 2018. PMID: 29197217
-
Human renal tubular cells contain CD24/CD133 progenitor cell populations: Implications for tubular regeneration after toxicant induced damage using cadmium as a model.Toxicol Appl Pharmacol. 2017 Sep 15;331:116-129. doi: 10.1016/j.taap.2017.05.038. Epub 2017 Jun 3. Toxicol Appl Pharmacol. 2017. PMID: 28587817 Free PMC article.
-
The RPTEC/TERT1 Cell Line as an Improved Tool for In Vitro Nephrotoxicity Assessments.Biol Trace Elem Res. 2015 Jul;166(1):66-71. doi: 10.1007/s12011-015-0339-y. Epub 2015 Apr 19. Biol Trace Elem Res. 2015. PMID: 25893367 Free PMC article. Review.
-
Kidney-on-a-chip technology for renal proximal tubule tissue reconstruction.Eur J Pharmacol. 2016 Nov 5;790:46-56. doi: 10.1016/j.ejphar.2016.07.018. Epub 2016 Jul 9. Eur J Pharmacol. 2016. PMID: 27401035 Review.
References
-
- Zhang B., Korolj A., Lai B.F.L., Radisic M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018;3:257–278. doi: 10.1038/s41578-018-0034-7. - DOI
-
- King S.M., Higgins J.W., Nino C.R., Smith T.R., Paffenroth E.H., Fairbairn C.E., Docuyanan A., Shah V.D., Chen A.E., Presnell S.C., et al. 3D Proximal Tubule Tissues Recapitulate Key Aspects of Renal Physiology to Enable Nephrotoxicity Testing. Front. Physiol. 2017;8:123. doi: 10.3389/fphys.2017.00123. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

