A Novel Device for the Evaluation of In Vitro Bacterial Colonization in Membranes for Guided Tissue and Bone Regeneration
- PMID: 39056989
- PMCID: PMC11275268
- DOI: 10.3390/dj12070202
A Novel Device for the Evaluation of In Vitro Bacterial Colonization in Membranes for Guided Tissue and Bone Regeneration
Abstract
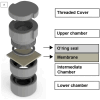
Purpose: To evaluate, in vitro, the efficiency of a novel apparatus to test the adherence and penetration of bacteria on different membranes for guided regeneration. Methodology: To create the 3D device, Computer Aided Design/Computer Aided Manufacturing (CAD/CAM) systems were used. Three types of biomaterials were tested (n = 6): (DT) a collagen membrane; (DS) a polymer membrane; and (LP) a dense polytetrafluoroethylene barrier. The biomaterials were adapted to the apparatuses and challenged with two different monospecies bacterial culture of A. actinomycetemcomitans b and S. mutans. After 2 h, bacterial adherence and penetration were quantified by counting the number of colony-forming units (CFUs). Two specimens from each group were used for image analysis using Confocal Laser Scanning Microscopy. Statistical analysis was performed. Findings: The DS group had a higher adherence of S. mutans compared to A. actinomycetemcomitans b (p = 0.05). There was less adherence of A. actinomycetemcomitans b in the DS group, compared to the LP (p = 0.011) and DT (p < 0.001) groups. Only the membranes allowed penetration, which was blocked by barriers. The DT group allowed a greater penetration of S. mutans to occur compared to A. actinomycetemcomitans b (p = 0.009), which showed a higher penetration into the DS membranes compared to S. mutans (p = 0.016). The penetration of A. actinomycetemcomitans b through DS was higher compared to its penetration through DT and LP (p < 0.01 for both). DT and DS allowed a greater penetration of S. mutans to occur compared to LP, which prevented both bacterial species from penetrating. Conclusion: The apparatus allowed for the settlement and complete sealing of the biomaterials, enabling standardization.
Keywords: 3D; CAD/CAM; bacteria; guided bone regeneration; guided tissue regeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Tumedei M., Mourão C.F., D’Agostino S., Dolci M., Di Cosola M., Piattelli A., Lucchese A. Histological and Histomorphometric Effectiveness of Barrier Membranes for Jawbone Regeneration: An Overview of More Than 30 Years’ Experience of Research Results of the Italian Implant Retrieval Center (1988–2020) Appl. Sci. 2021;11:2438. doi: 10.3390/app11052438. - DOI
-
- Wang H.L., Carroll M.J. Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int. 2001;32:504–515. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

