Canadian Consensus for Treatment of BRAF V600E Mutated Pediatric and AYA Gliomas
- PMID: 39057171
- PMCID: PMC11276207
- DOI: 10.3390/curroncol31070299
Canadian Consensus for Treatment of BRAF V600E Mutated Pediatric and AYA Gliomas
Abstract
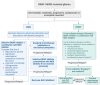
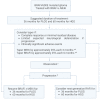
Background: The treatment of BRAF V600E gliomas with BRAF inhibitors (BRAFis) and MEK inhibitors (MEKis) has been increasingly integrated into clinical practice for pediatric low-grade gliomas (PLGGs) and pediatric high-grade gliomas (HGGs). However, some questions remain unanswered, such as the best time to start targeted therapy, duration of treatment, and discontinuation of therapy. Given that no clinical trial has been able to address these critical questions, we developed a Canadian Consensus statement for the treatment of BRAF V600E mutated pediatric as well as adolescent and young adult (AYA) gliomas. Methods: Canadian neuro-oncologists were invited to participate in the development of this consensus. The consensus was discussed during monthly web-based national meetings, and the algorithms were revised until a consensus was achieved. Results: A total of 26 participants were involved in the development of the algorithms. Two treatment algorithms are proposed, one for the initiation of treatment and one for the discontinuation of treatment. We suggest that most patients with BRAF V600E gliomas should be treated with BRAFis ± MEKis upfront. Discontinuation of treatment can be considered in certain circumstances, and we suggest a slow wean. Conclusions: Based on expert consensus in Canada, we developed algorithms for treatment initiation of children and AYA with BRAF V600E gliomas as well as a discontinuation algorithm.
Keywords: BRAF V600E mutation; BRAF inhibitor; MEK inhibitor; glioma; high-grade glioma; pediatric low-grade glioma.
Conflict of interest statement
JH is on the advisory board for Alexion, Astra Zeneca. SP is on the advisory board for Bayer, Alexion, AstraZeneca, and Esai, and received research funding from Novartis, Roche, Bayer, and SpringWorks Therapeutics.
Figures
References
-
- Ater J.L., Zhou T., Holmes E., Mazewski C.M., Booth T.N., Freyer D.R., Lazarus K.H., Packer R.J., Prados M., Sposto R., et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. - DOI - PMC - PubMed
-
- Lassaletta A., Scheinemann K., Zelcer S.M., Hukin J., Wilson B.A., Jabado N., Carret A.S., Lafay-Cousin L., Larouche V., Hawkins C.E., et al. Phase II Weekly Vinblastine for Chemotherapy-Naive Children with Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J. Clin. Oncol. 2016;34:3537–3543. doi: 10.1200/JCO.2016.68.1585. - DOI - PubMed
-
- Ostrom Q.T., Price M., Neff C., Cioffi G., A Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24((Suppl. S5)):v1–v95. doi: 10.1093/neuonc/noac202. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

