Enhancement of Biocompatibility of High-Transparency Zirconia Abutments with Human Gingival Fibroblasts via Cold Atmospheric Plasma Treatment: An In Vitro Study
- PMID: 39057321
- PMCID: PMC11277629
- DOI: 10.3390/jfb15070200
Enhancement of Biocompatibility of High-Transparency Zirconia Abutments with Human Gingival Fibroblasts via Cold Atmospheric Plasma Treatment: An In Vitro Study
Abstract
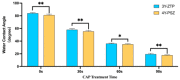
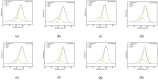
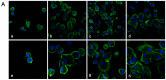
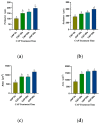
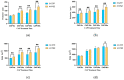
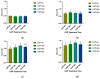
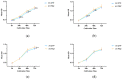
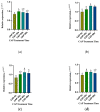
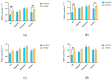
The objective of this study was to explore the effects of cold atmospheric plasma (CAP) treatment on the biological behavior of human gingival fibroblasts (HGFs) cultured on the surface of high-transparency zirconia. Two types of zirconia, 3Y-ZTP and 4Y-PSZ, were subjected to a CAP treatment for various treatment durations. Analyses of the physical and chemical properties of 3Y-ZTP and 4Y-PSZ were conducted using scanning electron microscopy, contact angle measurements, and X-ray photoelectron spectroscopy, both before and after CAP treatment. The biological responses of HGFs on both surfaces were assessed using CCK-8 assay, confocal laser scanning microscopy, and real-time PCR. Initially, the oxygen and hydroxyl contents on the surface of 4Y-PSZ exceeded those on 3Y-ZTP. CAP treatment enhanced the surface hydrophilicity and the reactive oxygen species (ROS) content of 4Y-PSZ, while not altering the surface morphology. After CAP treatment, HGFs' adhesion on 4Y-PSZ was superior, with more pronounced effects compared to 3Y-ZTP. Notably, HGFs counts and the expression of adhesion-related genes on 4Y-PSZ peaked following the CAP exposures for 30 s and 60 s. Consequently, this study demonstrates that, following identical CAP treatments, 4Y-PSZ is more effective in promoting HGFs adhesion compared to traditional 3Y-ZTP zirconia.
Keywords: abutment surface modification; cold atmosphere plasma; high-transparency zirconia; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Mechanical behavior and microstructural characterization of different zirconia polycrystals in different thicknesses.J Adv Prosthodont. 2021 Dec;13(6):385-395. doi: 10.4047/jap.2021.13.6.385. Epub 2021 Dec 22. J Adv Prosthodont. 2021. PMID: 35003554 Free PMC article.
-
[Effect of different plasma treated zirconia on the adhensive behaviour of human gingival fibroblasts].Beijing Da Xue Xue Bao Yi Xue Ban. 2019 Apr 18;51(2):315-320. doi: 10.19723/j.issn.1671-167X.2019.02.022. Beijing Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 30996375 Free PMC article. Chinese.
-
Surface roughness and optical characteristics evaluations after chairside adjustment of different zirconia types.J Esthet Restor Dent. 2024 Jul;36(7):1075-1080. doi: 10.1111/jerd.13246. Epub 2024 May 8. J Esthet Restor Dent. 2024. PMID: 38716797
-
Effect of Al2O3 Sandblasting Particle Size on the Surface Topography and Residual Compressive Stresses of Three Different Dental Zirconia Grades.Materials (Basel). 2021 Jan 28;14(3):610. doi: 10.3390/ma14030610. Materials (Basel). 2021. PMID: 33525702 Free PMC article.
-
Ultrashort pulse laser patterning of zirconia (3Y-TZP) for enhanced adhesion to resin-matrix cements used in dentistry: An integrative review.J Mech Behav Biomed Mater. 2023 Jul;143:105943. doi: 10.1016/j.jmbbm.2023.105943. Epub 2023 May 29. J Mech Behav Biomed Mater. 2023. PMID: 37276650 Review.
Cited by
-
Research of Optical Properties and Biocompatibility in Different Zones of Multilayered Translucent Zirconia on Hydrothermal Aging.Materials (Basel). 2024 Oct 24;17(21):5189. doi: 10.3390/ma17215189. Materials (Basel). 2024. PMID: 39517464 Free PMC article.
References
-
- Buser D., Janner S.F., Wittneben J.G., Bragger U., Ramseier C.A., Salvi G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat. Res. 2012;14:839–851. doi: 10.1111/j.1708-8208.2012.00456.x. - DOI - PubMed
-
- Palombo D., Rahmati M., Vignoletti F., Sanz-Esporrin J., Haugen H.J., Sanz M. Hard and soft tissue healing around implants with a modified implant neck configuration: An experimental in vivo preclinical investigation. Clin. Oral Implant. Res. 2021;32:1127–1141. doi: 10.1111/clr.13812. - DOI - PMC - PubMed
-
- Borie M., Lecloux G., Bosshardt D., Barrantes A., Haugen H.J., Lambert F., Bacevic M. Peri-implant soft tissue integration in humans—Influence of materials: A study protocol for a randomised controlled trial and a pilot study results. Contemp. Clin. Trials Commun. 2020;19:100643. doi: 10.1016/j.conctc.2020.100643. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

