Protective Effect of Indole-3-Aldehyde in Murine COVID-19-Associated Pulmonary Aspergillosis
- PMID: 39057395
- PMCID: PMC11278170
- DOI: 10.3390/jof10070510
Protective Effect of Indole-3-Aldehyde in Murine COVID-19-Associated Pulmonary Aspergillosis
Abstract
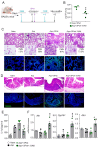
Aspergillus fumigatus is an environmental fungus recently included in the fungal high-priority pathogens by the World Health Organization. While immunodeficiency and/or pre-existing lung damage represent a well-recognized fertile ground for fungal growth, it is increasingly being recognized that severe viral infections may similarly favor A. fumigatus colonization and infection, as recently experienced in the Coronavirus disease 2019 (COVID-19) pandemic. Herein, in a murine model of COVID-19-associated pulmonary aspergillosis (CAPA), obtained by the concomitant exposure to the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike protein and A. fumigatus conidia, we found that the microbial compound indole-3-aldehyde (3-IAld) was able to ameliorate CAPA by working at multiple levels during viral infection and fungal superinfection, including epithelial barrier protection, promotion of antiviral responses, and limiting viral replication. As a consequence, 3-IAld limited the pathogenic sequelae of fungal superinfection as revealed by the controlled fungal burden and restrained inflammatory pathology. These results point to indole compounds as potential agents to prevent CAPA.
Keywords: CAPA; SARS-CoV-2; aspergillosis; indole-3-aldehyde.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- World Health Organization . WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Organización Mundial de la Salud (OMS); Geneva, Switzerland: 2022. 9240060251.
-
- Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., Klimko N., Lass-Florl C., Oladele R.O., Vinh D.C., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

