Biodegradable Cassava Starch/Phosphorite/Citric Acid Based Hydrogel for Slow Release of Phosphorus: In Vitro Study
- PMID: 39057454
- PMCID: PMC11276383
- DOI: 10.3390/gels10070431
Biodegradable Cassava Starch/Phosphorite/Citric Acid Based Hydrogel for Slow Release of Phosphorus: In Vitro Study
Abstract
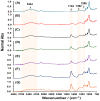
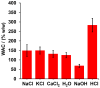
Phosphorous (P) is one the most important elements in several biological cycles, and is a fundamental component of soil, plants and living organisms. P has a low mobility and is quickly adsorbed on clayey soils, limiting its availability and absorption by plants. Here, biodegradable hydrogels based on Cassava starch crosslinked with citric acid (CA) were made and loaded with KH2PO4 and phosphorite to promote the slow release of phosphorus, the storing of water, and the reduction in P requirements during fertilization operations. Crosslinking as a function of CA concentrations was investigated by ATR-FTIR and TGA. The water absorption capacity (WAC) and P release, under different humic acid concentration regimens, were studied by in vitro tests. It is concluded that hydrogel formed from 10% w/w of CA showed the lowest WAC because of a high crosslinking degree. Hydrogel containing 10% w/w of phosphorite was shown to be useful to encouraging the slow release of P, its release behavior being fitted to the Higuchi kinetics model. In addition, P release increased as humic acid contents were increased. These findings suggest that these hydrogels could be used for encouraging P slow release during crop production.
Keywords: Cassava starch; P slow release; hydrogels; phosphorite.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Rashid A., Schutte B.J., Ulery A., Deyholos M.K., Sanogo S., Lehnhoff E.A., Beck L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy. 2023;13:1521. doi: 10.3390/agronomy13061521. - DOI
-
- Savci S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012;3:73. doi: 10.7763/IJESD.2012.V3.191. - DOI
-
- Schoumans O.F., Chardon W.J., Bechmann M.E., Gascuel-Odoux C., Hofman G., Kronvang B., Dorioz J.M. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Sci. Total Environ. 2014;468:1255–1266. doi: 10.1016/j.scitotenv.2013.08.061. - DOI - PubMed
-
- Sigurdarson J.J., Svane S., Karring H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018;17:241–258. doi: 10.1007/s11157-018-9466-1. - DOI
-
- Skrzypczak D., Witek-Krowiak A., Dawiec-Liśniewska A., Podstawczyk D., Mikula K., Chojnacka K. Immobilization of biosorbent in hydrogel as a new environmentally friendly fertilizer for micronutrients delivery. J. Clean. Prod. 2019;241:118387. doi: 10.1016/j.jclepro.2019.118387. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

