ER-transiting bacterial toxins amplify STING innate immune responses and elicit ER stress
- PMID: 39057915
- PMCID: PMC11321001
- DOI: 10.1128/iai.00300-24
ER-transiting bacterial toxins amplify STING innate immune responses and elicit ER stress
Abstract
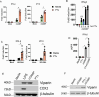
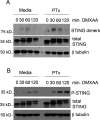
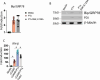
The cGAS/STING sensor system drives innate immune responses to intracellular microbial double-stranded DNA (dsDNA) and bacterial cyclic nucleotide second messengers (e.g., c-di-AMP). STING-dependent cell-intrinsic responses can increase resistance to microbial infection and speed pathogen clearance. Correspondingly, STING activation and signaling are known to be targeted for suppression by effectors from several bacterial pathogens. Whether STING responses are also positively regulated through sensing of specific bacterial effectors is less clear. We find that STING activation through dsDNA, by its canonical ligand 2'-3' cGAMP, or the small molecule DMXAA is potentiated following intracellular delivery of the AB5 toxin family member pertussis toxin from Bordetella pertussis or the B subunit of cholera toxin from Vibrio cholerae. Entry of pertussis toxin or cholera toxin B into mouse macrophages triggers markers of endoplasmic reticulum (ER) stress and enhances ligand-dependent STING responses at the level of STING receptor activation in a manner that is independent of toxin enzymatic activity. Our results provide an example in which STING responses integrate information about the presence of relevant ER-transiting bacterial toxins into the innate inflammatory response and may help to explain the in vivo adjuvant effects of catalytically inactive toxins.
Keywords: ADP ribosylation; GPCR; STING; cGAS; cholera toxin; endoplasmic reticulum; pertussis toxin; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- R21AI152051,R21AI151352/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R21 AI152051/AI/NIAID NIH HHS/United States
- CH-649-CRF/The State of Maryland | Maryland Department of Health (DHMH)
- IRG-18-160-16./American Cancer Society (ACS)
- R21 AI151352/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

