An Improved Postprocessing Method to Mitigate the Macroscopic Cross-Slice B0 Field Effect on R2* Measurements in the Mouse Brain at 7T
- PMID: 39058053
- PMCID: PMC11280969
- DOI: 10.3390/tomography10070081
An Improved Postprocessing Method to Mitigate the Macroscopic Cross-Slice B0 Field Effect on R2* Measurements in the Mouse Brain at 7T
Abstract
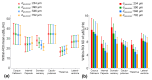
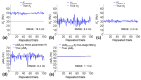
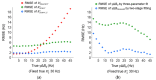
The MR transverse relaxation rate, R2*, has been widely used to detect iron and myelin content in tissue. However, it is also sensitive to macroscopic B0 inhomogeneities. One approach to correct for the B0 effect is to fit gradient-echo signals with the three-parameter model, a sinc function-weighted monoexponential decay. However, such three-parameter models are subject to increased noise sensitivity. To address this issue, this study presents a two-stage fitting procedure based on the three-parameter model to mitigate the B0 effect and reduce the noise sensitivity of R2* measurement in the mouse brain at 7T. MRI scans were performed on eight healthy mice. The gradient-echo signals were fitted with the two-stage fitting procedure to generate R2corr_t*. The signals were also fitted with the monoexponential and three-parameter models to generate R2nocorr* and R2corr*, respectively. Regions of interest (ROIs), including the corpus callosum, internal capsule, somatosensory cortex, caudo-putamen, thalamus, and lateral ventricle, were selected to evaluate the within-ROI mean and standard deviation (SD) of the R2* measurements. The results showed that the Akaike information criterion of the monoexponential model was significantly reduced by using the three-parameter model in the selected ROIs (p = 0.0039-0.0078). However, the within-ROI SD of R2corr* using the three-parameter model was significantly higher than that of the R2nocorr* in the internal capsule, caudo-putamen, and thalamus regions (p = 0.0039), a consequence partially due to the increased noise sensitivity of the three-parameter model. With the two-stage fitting procedure, the within-ROI SD of R2corr* was significantly reduced by 7.7-30.2% in all ROIs, except for the somatosensory cortex region with a fast in-plane variation of the B0 gradient field (p = 0.0039-0.0078). These results support the utilization of the two-stage fitting procedure to mitigate the B0 effect and reduce noise sensitivity for R2* measurement in the mouse brain.
Keywords: B0 inhomogeneity; R 2 *; T 2 *; background gradients; brain; gradient-echo; iron; myelin; noise; post-processing; quantitative MRI.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Deistung A., Schäfer A., Schweser F., Biedermann U., Turner R., Reichenbach J.R. Toward in vivo histology: A comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage. 2013;65:299–314. doi: 10.1016/j.neuroimage.2012.09.055. - DOI - PubMed
-
- Antharam V., Collingwood J.F., Bullivant J.P., Davidson M.R., Chandra S., Mikhaylova A., Finnegan M.E., Batich C., Forder J.R., Dobson J. High field magnetic resonance microscopy of the human hippocampus in Alzheimer’s disease: Quantitative imaging and correlation with iron. Neuroimage. 2012;59:1249–1260. doi: 10.1016/j.neuroimage.2011.08.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

