Experimental Granulomatous Amebic Encephalitis Caused by Acanthamoeba castellanii
- PMID: 39058187
- PMCID: PMC11281140
- DOI: 10.3390/tropicalmed9070145
Experimental Granulomatous Amebic Encephalitis Caused by Acanthamoeba castellanii
Abstract
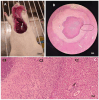
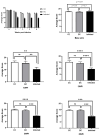
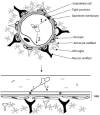
Acanthamoeba genus can affect humans with diseases such as granulomatous amebic encephalitis (GAE), a highly lethal neuroinfection. Several aspects of the disease still need to be elucidated. Animal models of GAE have advanced our knowledge of the disease. This work tested Wistar rats (Rattus norvegicus albinus) as an animal model of GAE. For this, 32 animals were infected with 1 × 106A. castellanii trophozoites of the T4 genotype. Ameba recovery tests were carried out using agar plates, vascular extravasation assays, behavioral tests, and histopathological technique with H/E staining. Data were subjected to linear regression analysis, one-way ANOVA, and Tukey's test, performed in the GraphPad Prism® 8.0 program, with a significance level of p < 0.05. The results revealed the efficiency of the model. Amebae were recovered from the liver, lungs, and brain of infected animals, and there were significant encephalic vascular extravasations and behavioral changes in these animals, but not in the control animals. However, not all infected animals showed positive histopathology for the analyzed organs. Nervous tissues were the least affected, demonstrating the role of the BBB in the defense of the CNS. Supported by the demonstrated evidence, we confirm the difficulties and the feasibilities of using rats as an animal model of GAE.
Keywords: Acanthamoeba; brain; diagnostic; encephalitis; experimental infection; rat.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Duggal S.D., Rongpharpi S.R., Duggal A.K., Kumar A., Biswal I. Role of Acanthamoeba in granulomatous encephalitis: A review. J. Infect. Dis. Immune Ther. 2017;1:1–7.
LinkOut - more resources
Full Text Sources

