Mechanisms Mediating the Combined Toxicity of Paraquat and Maneb in SH-SY5Y Neuroblastoma Cells
- PMID: 39058280
- PMCID: PMC11337211
- DOI: 10.1021/acs.chemrestox.3c00389
Mechanisms Mediating the Combined Toxicity of Paraquat and Maneb in SH-SY5Y Neuroblastoma Cells
Abstract
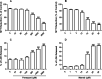
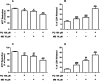
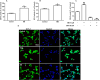
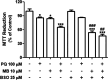
Epidemiological and experimental studies have demonstrated that combined exposure to the pesticides paraquat (PQ) and maneb (MB) increases the risk of developing Parkinson's disease. However, the mechanisms mediating the toxicity induced by combined exposure to these pesticides are not well understood. The aim of this study was to investigate the mechanism(s) of neurotoxicity induced by exposure to the pesticides PQ and MB isolated or in association (PQ + MB) in SH-SY5Y neuroblastoma cells. PQ + MB exposure for 24 and 48 h decreased cell viability and disrupted cell membrane integrity. In addition, PQ + MB exposure for 12 h decreased the mitochondrial membrane potential. PQ alone increased reactive oxygen species (ROS) and superoxide anion generation and decreased the activity of mitochondrial complexes I and II at 12 h of exposure. MB alone increased ROS generation and depleted intracellular glutathione (GSH) within 6 h of exposure. In contrast, MB exposure for 12 h increased the GSH levels, the glutamate cysteine ligase (GCL, the rate-limiting enzyme in the GSH synthesis pathway) activity, and increased nuclear Nrf2 staining. Pretreatment with buthionine sulfoximine (BSO, a GCL inhibitor) abolished the MB-mediated GSH increase, indicating that MB increases GSH synthesis by upregulating GCL, probably by the activation of the Nrf2/ARE pathway. BSO pretreatment, which did not modify cell viability per se, rendered cells more sensitive to MB-induced toxicity. In contrast, treatment with the antioxidant N-acetylcysteine protected cells from MB-induced toxicity. These findings show that the combined exposure of SH-SY5Y cells to PQ and MB induced a cytotoxic effect higher than that observed when cells were subjected to individual exposures. Such a higher effect seems to be related to additive toxic events resulting from PQ and MB exposures. Thus, our study contributes to a better understanding of the toxicity of PQ and MB in combined exposures.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

