Ipatasertib plus Paclitaxel for Patients with PIK3CA/AKT1/PTEN-Altered Locally Advanced Unresectable or Metastatic Triple-Negative Breast Cancer in the IPATunity130 Phase III Trial
- PMID: 39058425
- PMCID: PMC11443247
- DOI: 10.1158/1078-0432.CCR-24-0465
Ipatasertib plus Paclitaxel for Patients with PIK3CA/AKT1/PTEN-Altered Locally Advanced Unresectable or Metastatic Triple-Negative Breast Cancer in the IPATunity130 Phase III Trial
Abstract
Purpose: In the randomized phase II LOTUS trial, combining ipatasertib with first-line paclitaxel for triple-negative breast cancer (TNBC) improved progression-free survival (PFS), particularly in patients with PIK3CA/AKT1/PTEN-altered tumors. We aimed to validate these findings in a biomarker-selected TNBC population.
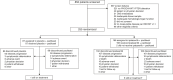
Patients and methods: In Cohort A of the randomized double-blind placebo-controlled phase III IPATunity130 trial, taxane-eligible patients with PIK3CA/AKT1/PTEN-altered measurable advanced TNBC and no prior chemotherapy for advanced disease were randomized 2:1 to ipatasertib (400 mg, days 1-21) or placebo, both plus paclitaxel (80 mg/m2, days 1, 8, and 15), every 28 days until disease progression or unacceptable toxicity. The primary endpoint was investigator-assessed PFS.
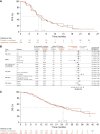
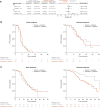
Results: Between February 2018 and April 2020, 255 patients were randomized (168 to ipatasertib, 87 to placebo). At the primary analysis, there was no significant difference between treatment arms in PFS [hazard ratio 1.02, 95% confidence interval (CI), 0.71-1.45; median 7.4 months with ipatasertib vs. 6.1 months with placebo]. The final analysis showed no difference in overall survival between treatment arms (hazard ratio 1.08, 95% CI, 0.73-1.58; median 24.4 vs. 24.9 months, respectively). Ipatasertib was associated with more grade ≥3 diarrhea (9% vs. 2%) and adverse events leading to dose reduction (39% vs. 14%) but similar incidences of grade ≥3 adverse events (51% vs. 46%). Exploratory subgroup analyses by PAM50 and Burstein gene expression showed inconsistent results.
Conclusions: Adding ipatasertib to paclitaxel did not improve efficacy in PIK3CA/AKT1/PTEN-altered advanced TNBC. Biomarkers for benefit from PI3K/AKT pathway inhibition in TNBC remain poorly understood.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
R.A. Dent reports travel sponsorship and/or honoraria as part of advisory board committees from AstraZeneca, MSD, Pfizer, Eisai, Novartis, Daiichi Sankyo, and Roche. S.-B. Kim reports research funding from Novartis, Sanofi-Aventis, and DongKook Pharm Co; consultancy in advisory boards for Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co. Ltd., ISU Abxis, OBI pharma, Beigene, and Daiichi Sankyo; and stocks in Genopeaks and NeogeneTC. M. Oliveira reports grants, personal fees, and nonfinancial support from Roche during the conduct of the study, as well as grants, personal fees, and nonfinancial support from AstraZeneca and Gilead; personal fees from Cureos Science, iTEOS, Lilly, MSD, Relay Therapeutics, and Daiichi Sankyo; grants and personal fees from Roche and Seagen; grants from Zenith Epigenetics, Ayala Pharmaceuticals, Genentech, and Immutep; and nonfinancial support from Eisai and Pierre-Fabre outside the submitted work. C. Barrios reports grants from Nektar, Pfizer, Polyphor, Amgen, Daiichi Sankyo, Sanofi, Exelixis, Regeneron, Novartis, GSK, Janssen, OBI Pharma, Lilly, Seagen, Roche, BMS, MSD, AstraZeneca, Novocure, Aveo Oncology, Takeda, PharmaMar, Gilead Sciences, Servier, Tolmar, Nanobiotix, Dizal Pharma, TRIO, Labcorp, ICON, IQVIA, Parexel, Nuvisan, PSI, Worldwide, Latinaba, Fortrea, PPD, and Syneos Health during the conduct of the study, as well as personal fees from Gilead, Boehringer Ingelheim, GSK, Novartis, Pfizer, Roche/Genentech, Eisai, Bayer, MSD, AstraZeneca, Zodiac, Lilly, Sanofi, Daiichi Sankyo, and Roche outside the submitted work; and stock ownership in Thummi and MedSIR. J. O’Shaughnessy reports personal fees from Agendia, Aptitude Health, Daiichi Sankyo, Eisai, G1 Therapeutics, Genentech, Gilead Sciences, Lilly, Loxo Oncology, Merck, Novartis, Pfizer, Pierre Fabre Pharmaceuticals, Puma Biotechnology, Roche, Samsung Bioepis, Ontada, Seagen, Stemline Therapeutics, AstraZeneca, Fishawack Health, and Veru outside the submitted work. S. Saji reports grants from AstraZeneca during the conduct of the study, as well as personal fees from Kyowa Kirin, Pfizer, Ono, Exact Sciences, and Nippon Kayaku; grants and personal fees from Taiho, MSD, Novartis, Eisai, Takeda, Daiichi Sankyo, Eli Lilly, Gilead, AstraZeneca, and Chugai; and grants from Sanofi outside the submitted work. M. Philco reports other support from Roche and Bayer outside the submitted work. D. Bradley reports other support from Roche Products Limited during the conduct of the study, as well as other support from Roche Products Limited outside the submitted work; in addition, D. Bradley has a patent for HER2 treatment pending. H. Hinton reports being an employee and shareholder of F. Hoffmann La-Roche. M.J. Wongchenko reports other support from Genentech/Roche during the conduct of the study, as well as other support from Genentech/Roche outside the submitted work. N. Turner reports advisory board honoraria from AstraZeneca, Lilly, Pfizer, Roche/Genentech, Novartis, GlaxoSmithKline, Repare Therapeutics, Relay Therapeutics, Gilead, Inivata, Guardant, and Exact Sciences and research funding from AstraZeneca, Pfizer, Roche/Genentech, Merck Sharpe and Dohme, Guardant Health, Invitae, Inivata, Personalis, and Natera. No disclosures were reported by the other authors.
Figures
References
-
- Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol 2008;8:393–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

