Reversing anxiety by targeting a stress-responsive signaling pathway
- PMID: 39058580
- PMCID: PMC11295078
- DOI: 10.1073/pnas.2400078121
Reversing anxiety by targeting a stress-responsive signaling pathway
Abstract
Current treatments of anxiety and depressive disorders are plagued by considerable side effects and limited efficacies, underscoring the need for additional molecular targets that can be leveraged to improve medications. Here, we have identified a molecular cascade triggered by chronic stress that exacerbates anxiety- and depressive-like behaviors. Specifically, chronic stress enhances Src kinase activity and tyrosine phosphorylation of calmodulin, which diminishes MyosinVa (MyoVa) interaction with Neuroligin2 (NL2), resulting in decreased inhibitory transmission and heightened anxiety-like behaviors. Importantly, pharmacological inhibition of Src reinstates inhibitory synaptic deficits and effectively reverses heightened anxiety-like behaviors in chronically stressed mice, a process requiring the MyoVa-NL2 interaction. These data demonstrate the reversibility of anxiety- and depressive-like phenotypes at both molecular and behavioral levels and uncover a therapeutic target for anxiety and depressive disorders.
Keywords: GABAergic synapses; Neuroligin2; Src kinase; anxiety; chronic stress.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


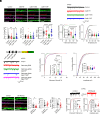
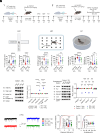
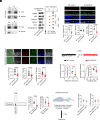
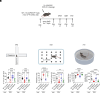

References
-
- Javaid S. F., et al. , Epidemiology of anxiety disorders: Global burden and sociodemographic associations. Middle East Curr. Psychiatry 30, 1–11 (2023).
-
- Castaldelli-Maia J. M., Bhugra D., Analysis of global prevalence of mental and substance use disorders within countries: Focus on sociodemographic characteristics and income levels. Int. Rev. Psychiatry 34, 6–15 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

